Nucleotide sequence and expression vector of encoded recombinant urate oxidase protein
A urate oxidase protein and nucleic acid sequence technology, applied in the field of protein engineering, can solve problems such as unfavorable treatment of leukemia, increase the burden on the kidney, reduce the curative effect, etc., and achieve the effects of easy quality control, small batch-to-batch variation, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
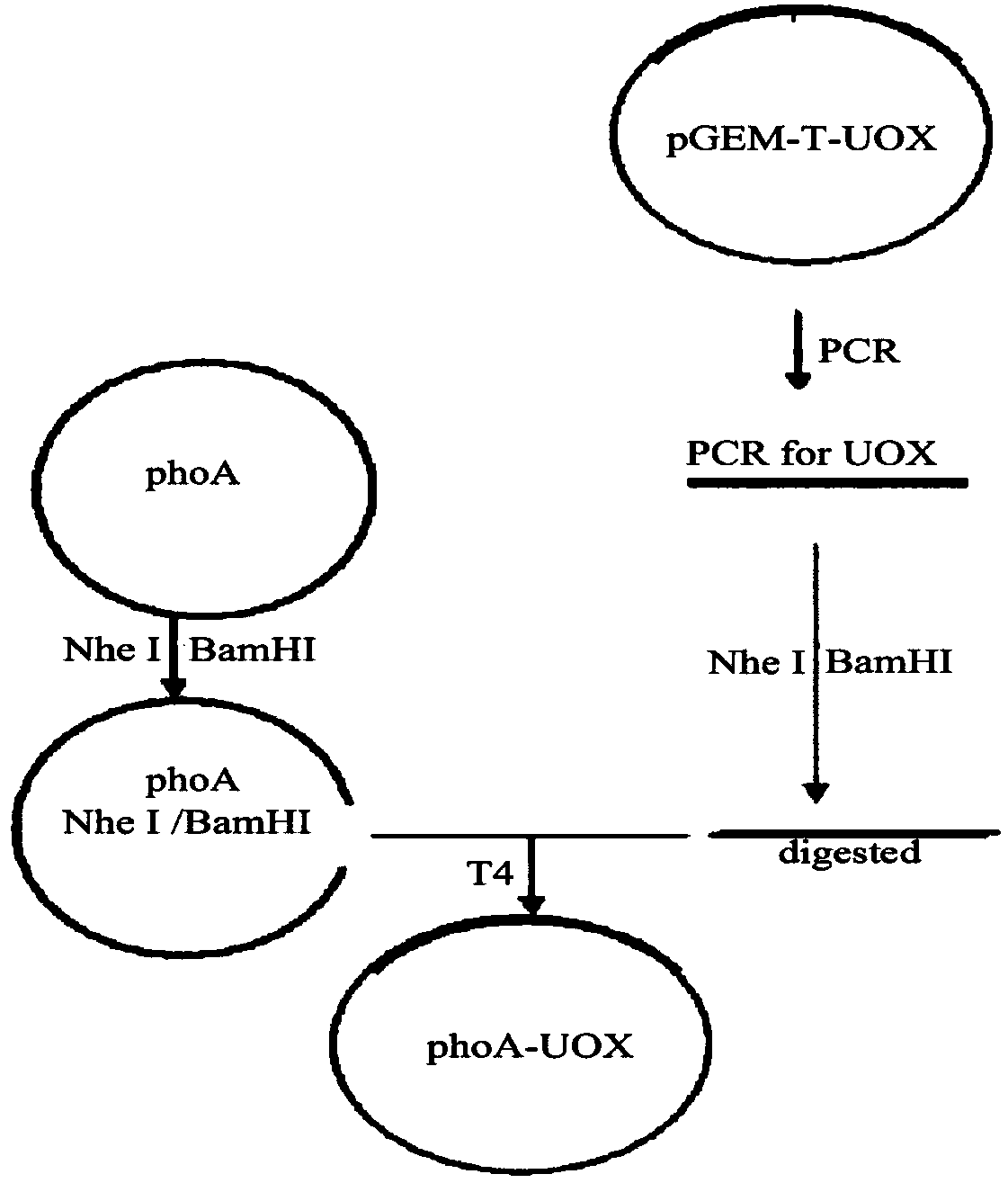
[0029] Construction of engineering bacteria expressing recombinant urate oxidase
[0030] 1. Target gene synthesis
[0031] According to the known natural amino acid sequence of recombinant urate oxidase, a specific recombinant urate oxidase nucleic acid sequence fragment was obtained through screening, according to the codon preference of Escherichia coli and considering the elimination of hairpin structures and other secondary structures that are not conducive to expression, Under the condition of not changing the amino acid sequence, the coding sequence primer of recombinant urate oxidase is designed, and its sequence is as follows.
[0032] Recombinant urate oxidase upstream primer:
[0033] 5'GATGAT GCTAGC ATG TCA ACA ACG CTC TCA 3'NheI (SEQ ID NO.2)
[0034] Downstream primers for recombinant urate oxidase:
[0035] 5'GATGAT GGATCC TTA CAA CTT GGT CTT CTC C 3'BamH I (SEQ ID NO.3)
[0036] The full-length sequence of recombinant urate oxidase was obtained by bridging ...
Embodiment 2
[0046] Study on High Density Fermentation of Engineering Bacteria
[0047] Configure low phosphorus medium: low phosphate medium contains 10g of peptone, 2.5g of yeast extract, MgSO in every 1000ml medium 4 ·7H 2O 0.5g, 1mol / L Tris-Cl (pH 8.0) 50ml, phenol red 2ml, glucose 10g.
[0048] Pick a single clone from the positive plate into a 250ml Erlenmeyer flask containing 50mI LB medium and culture overnight. The next day, inoculate the overnight cultured bacterial solution in the low-phosphorus medium at a ratio of 1:100.
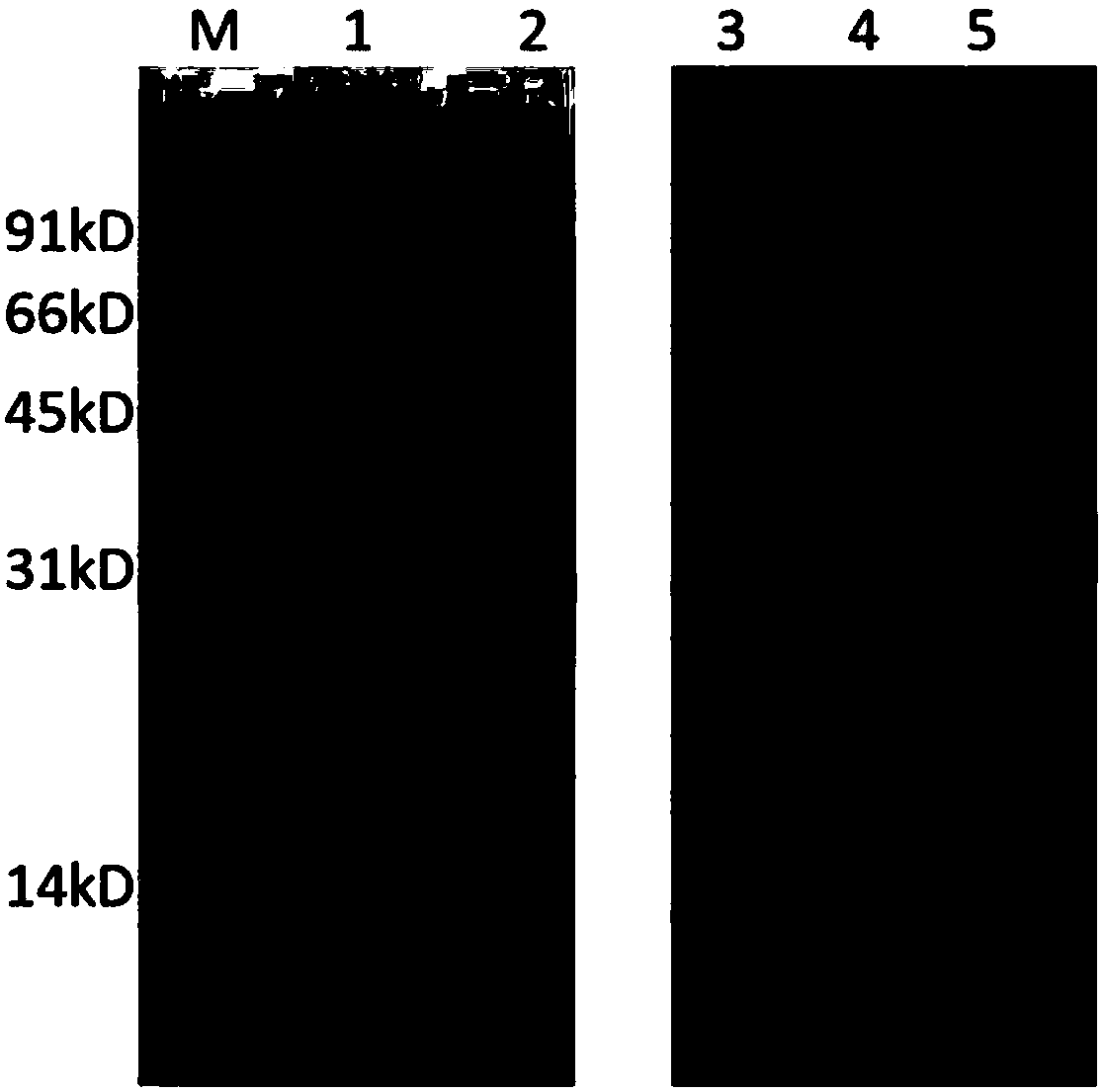
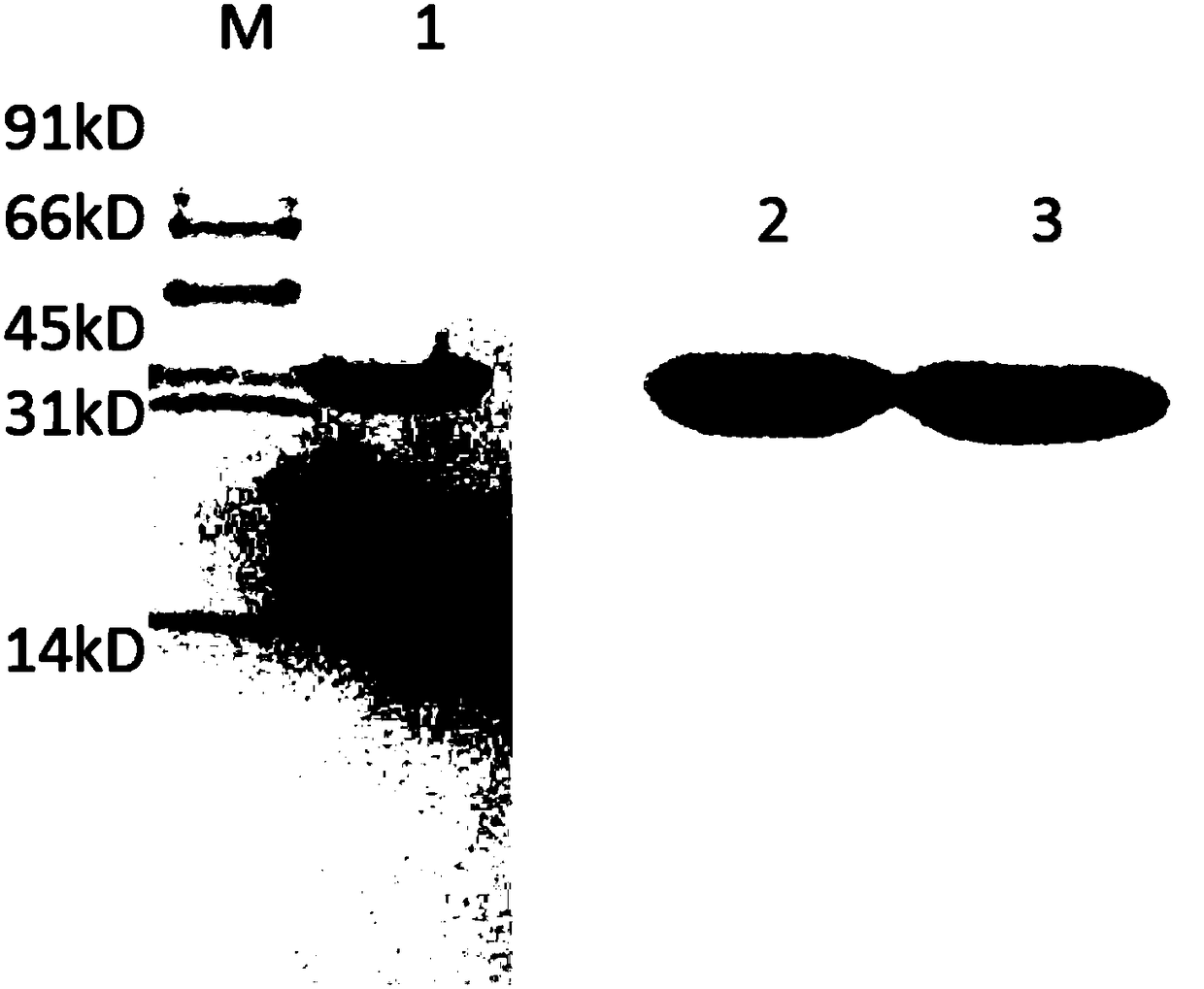
[0049] Appropriately add carbon source (such as glycerol) and nitrogen source (such as ammonia water) to adjust pH to 7.2-7.4, DO>50%, temperature at 30-32°C, and end of induction for 12 hours. The samples were detected by SDS-PAGE (and scanned) to determine the protein content. results, such as figure 2 As shown, the expression level of recombinant urate oxidase protein reached more than 20%.
[0050] After fermenting and expressing the recombinant u...
Embodiment 3
[0053] Purification of recombinant urate oxidase
[0054] Prepare TSE solution: 20mM Tris-Hcl, pH 7.0, 20% glucose, 1mM EDTA.
[0055] The fermentation broth was centrifuged at 5000 rpm for 10 min at 4°C to obtain bacterial cells. The bacterial cells were resuspended in pre-cooled TSE solution and thoroughly stirred evenly. Centrifuge at 12000rpm for 10min to obtain a precipitate. Add 20Mm, pH7.0 Tris-CL to resuspend, and stir well. Centrifuge at 12000rpm for 10min, and collect the supernatant. The result is as figure 2 Lane 3 shows.
[0056] Chromatography was performed three times.
[0057] 1. Ion exchange chromatography
[0058] Chromatography medium: DEAE chromatography column
[0059] Buffer: Solution A: PBS (pH7.4, 20mM)
[0060] Solution B: PBS (pH7.4, 20mM) + 1M NaCl
[0061] Loading: Load the TSE extraction solution.
[0062] Cleaning: Wash the column with solution A after loading the sample.
[0063] Gradient: Solution B was raised from 0% to 100% after w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com