Vaccine composition as well as preparation method and application thereof
一种疫苗组合物、减蛋综合征的技术,应用在生物化学设备和方法、疫苗、多价疫苗等方向,能够解决免疫效力低下、疫苗难以提供免疫效果、未开发产品等问题,达到生物安全性好、良好预防和控制效果、免疫原性好的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
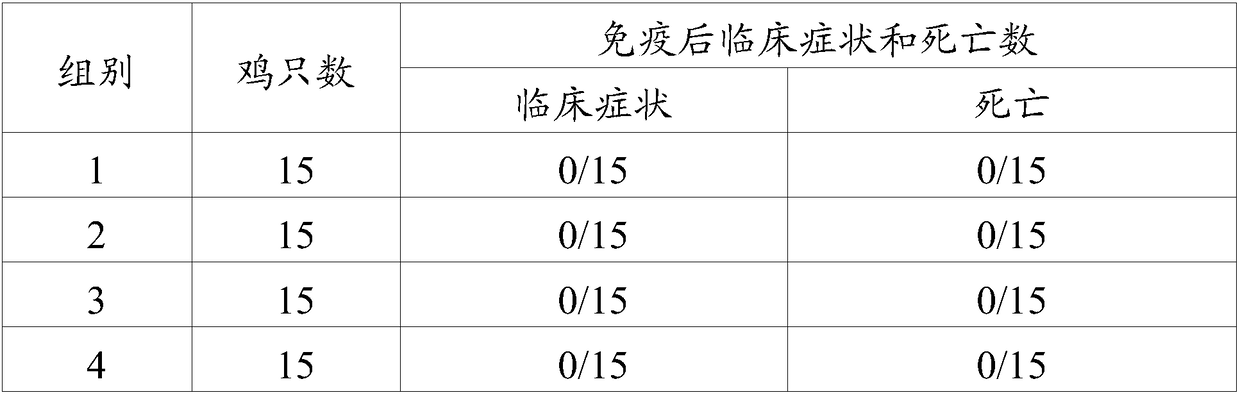
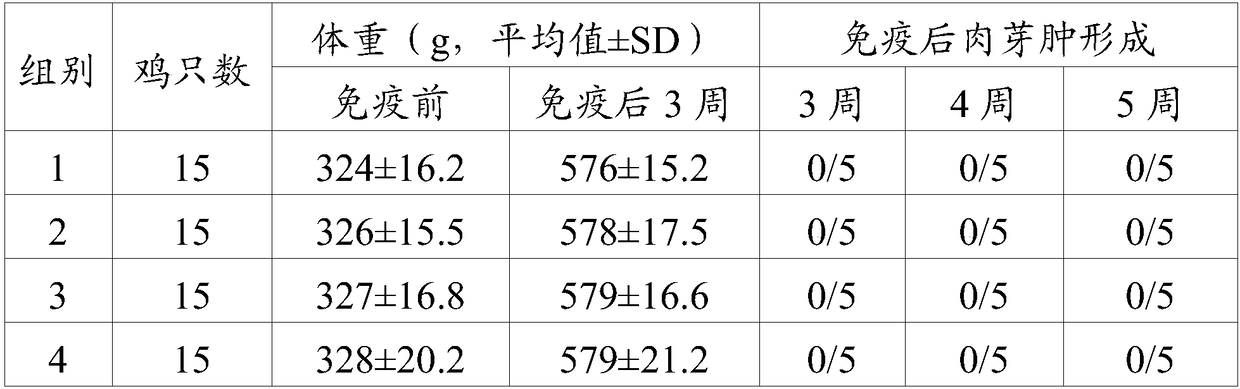
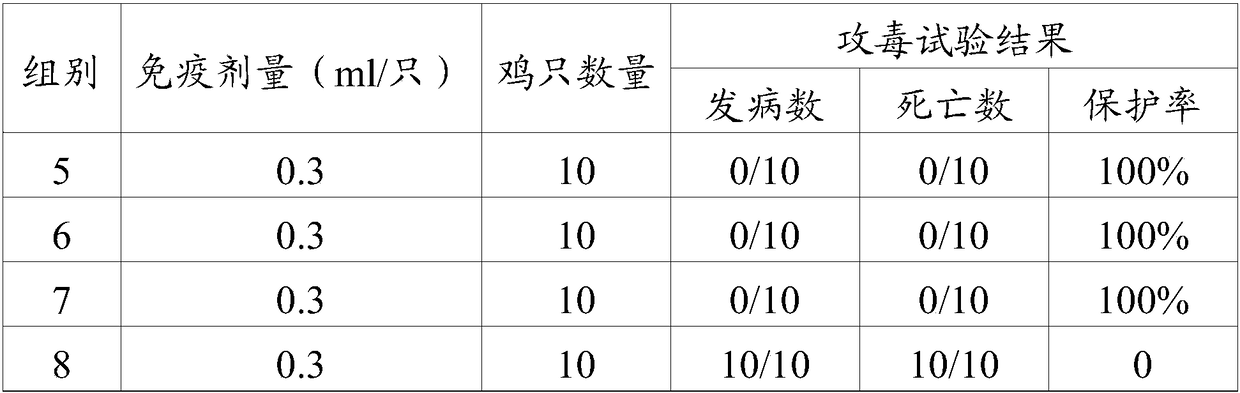
[0023] As an embodiment of the present invention, the avian adenovirus fiber-2 protein is the protein encoded by SEQ.ID NO.1.
[0024] As an embodiment of the present invention, the protein content of the avian adenovirus fiber-2 is AGP titer ≥ 1:2.
[0025] As a preferred embodiment of the present invention, the protein content of the avian adenovirus fiber-2 is AGP titer 1:2-1:16.
[0026]The term "veterinary acceptable carrier" refers to all other components in the vaccine composition of the present invention except the adenovirus antigen, a carrier or diluent that does not stimulate the body and does not hinder the use of the biological activity and properties of the compound, Adjuvants are preferred. The term "adjuvant" may include aluminum gel adjuvants; saponins, such as Quil A, QS-21 (Cambridge Biotech Incorporation, Cambridge MA), GPI-0100 (Galenica Pharmaceuticals Incorporation, Birmingham AL); water-in-oil emulsions; Oil emulsions; water-in-oil-in-water emulsions;...
Embodiment 1
[0060] Example 1 Construction of pET28a_FADV_fiber-2 expression vector
[0061] 1 Extraction of FADV virus DNA
[0062] The plasmid extraction kit was purchased from Tiangen Biotech; T4 DNA Ligase was purchased from BioLab; the pET28a plasmid was purchased from Novagen; the agarose gel recovery kit was purchased from Tianze Biotech, and other reagents were of analytical grade.
[0063] According to the instructions of the virus DNA extraction kit, take 0.2ml of infected avian adenovirus FAV-HN strain (fowl adenovirus, FAV-HN strain (Fowl aviadenovirus, strain FAV-HN), the preservation number is: CCTCC NO.V201609, and the depository unit is China Typical Culture Collection Center, the preservation address is Wuhan University, Wuhan, China, and the preservation time is February 29, 2016.) The chicken liver suspension is placed in a sterile 1.5ml centrifuge tube, and 0.4ml VB is added to the sample solution. Vortex to mix and let stand at room temperature for 10 minutes. Add 0....
Embodiment 2
[0071] Example 2 Preparation of fiber-2 protein
[0072] Inoculate the pET28a_FADV_fiber-2 / E.Coli BL21(DE3) strain containing prepared in Example 1 into the LB medium containing 50-100 μg / ml kanamycin, the inoculum size is 1% (V / V), 37 Cultivate with shaking. When OD600=0.4-0.6, place it at 28°C for 30 minutes. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to make the final concentration 0.1-1.0 mM, and cultured with shaking at 28° C. for 24 hours. After the cultivation, the cells were collected and resuspended with PBS (sodium chloride, 8g, potassium chloride, 0.2g, disodium hydrogen phosphate, 1.44g, potassium dihydrogen phosphate, 0.24g, adjusted to pH 7.4, constant volume 1L) Bacteria were sonicated and centrifuged to obtain the supernatant. The content of soluble target protein in the expression product is relatively high, the AGP titer of fiber-2 protein reaches 1:64, and the endotoxin content is 0.48×10 5 EU / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com