Pyronema omphalodes natural antibacterial peptides and application thereof
An antibacterial peptide, a natural technology, applied in the field of biomedicine, can solve the problem of high production cost of antibacterial peptides, and achieve the effect of significant bacteriostatic activity, good bactericidal effect, broad application value and market prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
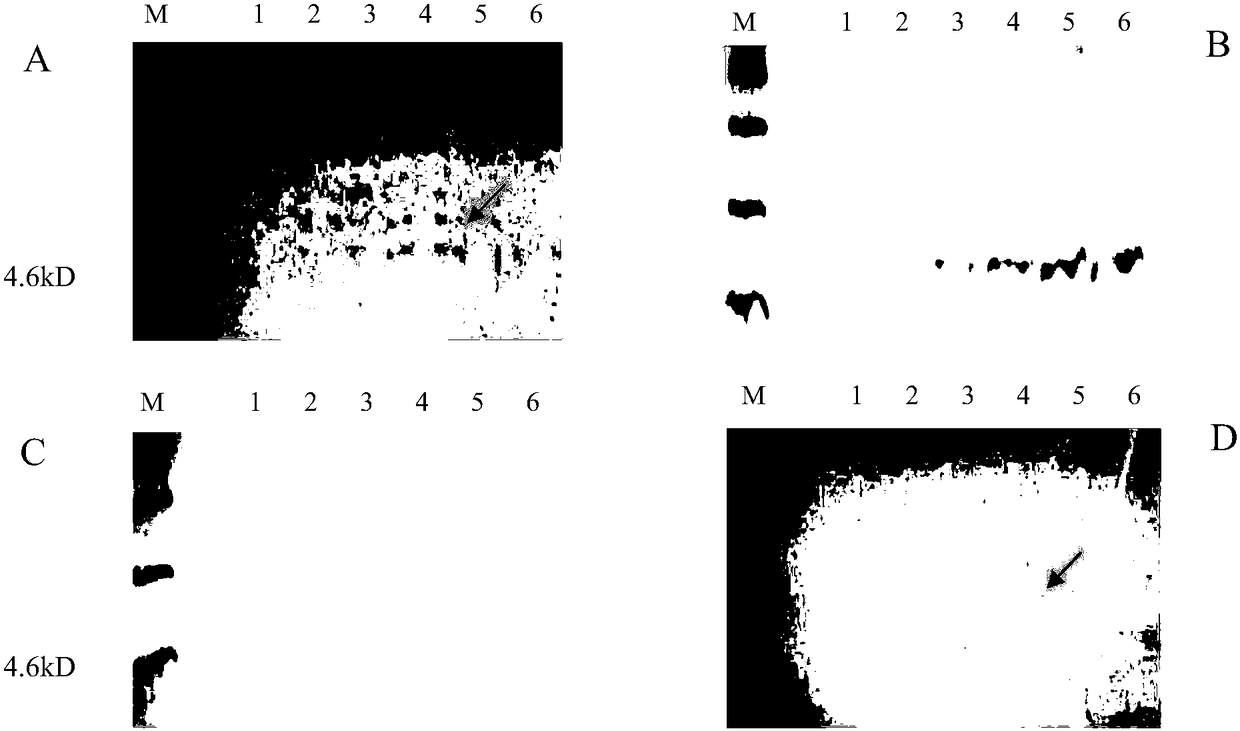
[0037] Example 1 Discovery of natural antimicrobial peptides and Plectasin similar to fungal defensins
[0038] Through searching, four polypeptide sequences (U4LUG1, U4LWE8, U4LNY7, U4LND7) derived from Pyronema omphalodes were found from the fungal proteome data of the UniProt database (http: / / www.uniprot.org / ). The group of polypeptide sequences and their functional regions are respectively shown in SEQ ID NO: 1-4 and SEQ ID NO: 5-8.
[0039]The group of polypeptide full-length gene sequences was compared with the blastX software (https: / / blast.ncbi.nlm.nih.gov / Blast.cgi?PROGRAM=blastx&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) on the NCBI website, and the results showed that, The encoded product of this gene may be the precursor of fungal defensin family antimicrobial peptide. The sequence of the polypeptide precursor coded by this group of genes was further compared with the protein blast software (https: / / blast.ncbi.nlm.nih.gov / Blast.cgi?PROGRAM=blastp&PAGE_TY PE=BlastSe...
Embodiment 2
[0040] Embodiment 2 antimicrobial peptide P1-P4 gene synthesis
[0041] Antimicrobial peptide P1-P4 gene sequences (SEQ ID NO:9-12) were designed according to the codon preference of Pichia pastoris. In order to ensure the integrity of the sequence during expression, an XhoI restriction site and a Kex2 restriction site were added to the 5' end of the mutant gene sequence, and a TAA, TAG terminator sequence and XbaI restriction site were added to the 3' end. The above sequence was completed by Shanghai Sangon Bioengineering Co., Ltd.
Embodiment 3
[0042] Example 3 Construction of Pichia pastoris inducible expression vectors pPICP1-pPICP4
[0043] The synthetic gene fragment and vector pPICZαA were double-digested with restriction endonucleases XhoI and XbaI respectively, the vector fragment of pPICZαA and the antimicrobial peptide gene fragment were recovered, and ligated to obtain vectors pPICP1-pPICP4;
[0044] Detailed construction process of vectors pPICP1~pPICP4: use restriction endonucleases XhoI and XbaI to carry out double enzyme digestion on the synthesized gene fragment and pPICZαA respectively. The enzyme digestion system and conditions are as follows:
[0045]
[0046] After adding the above enzyme digestion system, react at 37°C for 3 hours, and detect by 2% agarose gel electrophoresis, electrophoresis conditions: 130V, 30min. After electrophoresis, use a scalpel to cut the electrophoretic bands corresponding to the carrier fragment and the gene fragment under the ultraviolet light, and use the Tiangen B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com