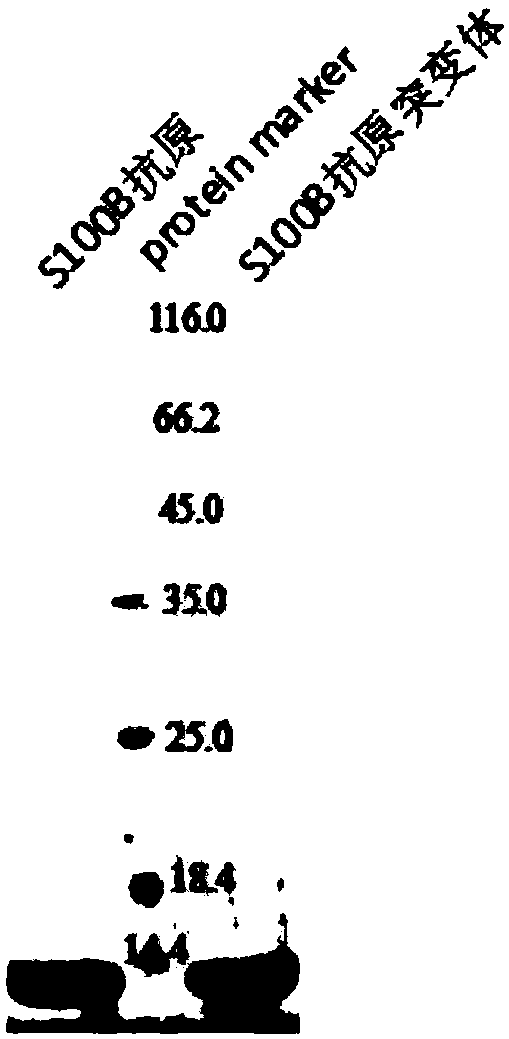S100B mutant and application thereof
A mutant and protein technology, applied in the field of protein engineering, can solve the problems of low yield and difficult antibody elution, and achieve the effects of high purity, easy elution and separation, and improved recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Construction of S100B Gene Mutation Library
[0015] S100B抗原(购自上海友科生物科技有限公司,人工合成)的氨基酸序列如表SEQID No:1所示,即:MSELEKAMVALIDVFHQYSGREGDKHKLKKSELKELINNELSHFLEEIKEQEVVDKVMETLDNDGDGECDFQEFMAFVAMVTTACHEFFEHE;其碱基序列如表SEQ ID No:2所示,即:ATGTCTGAGCTGGAGAAGGCCATGGTGGCCCTCATCGACGTTTTCCACCAATATTCTGGAAGGGAGGGAGACAAGCACAAGCTGAAGAAATCCGAACTCAAGGAGCTCATCAACAATGAGCTTTCCCATTTCTTAGAGGAAATCAAAGAGCAGGAGGTTGTGGACAAAGTCATGGAAACACTGGACAATGAT。
[0016] In order to solve the binding force of the S100B antigen (the amino acid sequence is shown in table SEQ ID No: 1, and the nucleotide sequence is shown in table SEQ ID No: 2), the protein was screened for a large number of mutations by directed evolution technology, and optimized And design a pair of PCR primers:
[0017] The upstream primer is as described in the sequence table SEQ ID No: 3, namely: 5'-GGGAGGGAGACAAGCACAGG-3';
[0018] The downstream primer is as described in SEQ ID No: 4 in the sequence table, namely: 5'-GTTCGGATTTCTTCAGCCTGTG...
Embodiment 2
[0019] Construction and fermentation of embodiment 2 S100B mutant expression strain
[0020] 1. Use the S100B gene as a template, use the above primers, and add 2×TransStart FastPfu PCRSuperMix to amplify the entire plasmid gene. The PCR reaction conditions were: denaturation at 94°C for 5 minutes; then denaturation at 94°C for 30 seconds, renaturation at 60°C for 30 seconds, extension at 72°C for 5 minutes, after 30 cycles, full extension at 72°C for 10 minutes, and storage at 4°C. After PCR, add DMT enzyme to digest and degrade the non-mutant plasmid template in vitro, and recover the target gene by gel.
[0021] 2. Digest the gel-recovered mutant gene and pET-28a vector with BamHI and XhoI to expose the same ends, connect with T4 ligase at room temperature, transform into DMT competent cells, coat LB+Kan plates, and culture overnight at 37°C . The next day, the recombinant strains were screened.
[0022] 3. Pick a monoclonal colony from the culture plate and inoculate it...
Embodiment 3
[0027] Embodiment 3 S100B mutant purification
[0028] Buffer A: 50mM PBS, 500mM NaCl
[0029] Buffer B: 50mM PBS, 500mM NaCl, 500mM imidazole
[0030] 1. After the induced expression, the bacterial solution was centrifuged at 8000rpm for 5min, resuspended in buffer A, ultrasonicated for 30min, and then centrifuged at 12000rpm for 30min to collect the supernatant.
[0031] 2. Add the supernatant to Ni-NTA for 1 hour, use buffer A and buffer B to prepare the imidazole concentration of 10mM, 20mM, 50mM, 200mM buffer to elute the target protein step by step.
[0032] 3. Start the peristaltic pump with a flow rate of 30rpm, turn on the protein detector at the same time, and use various gradient buffer solutions to reach the baseline of the detector until it is stable.
[0033] 4. Collect protein samples from various gradient elutions, and take a small amount of samples to prepare the samples required for SDS-PAGE.
[0034] 5. Collect the target protein, concentrate and change the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com