Nitrogen-doped carbon nano-array/ cobalt ferrite material
A nano-array, nitrogen-doped carbon technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of poor electrical conductivity of ferrite, and achieve the effects of low cost, high catalytic efficiency, and poor electrical conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of nitrogen-doped carbon nanoarray / cobalt ferrite material.
[0033] 1.1 Preparation of carbon paper
[0034] Take an appropriate amount of water and put it into a large beaker, put the prepared carbon paper into a 50mL beaker, take 20mL of ultrapure water into the beaker, and then take 1.345mL of HNO with a mass fraction of 68%. 3 Slowly add to the beaker. Put the small beaker into the beaker, put it on a magnetic stirrer for heating in a water bath, boil the carbon paper with acid at 90°C for 1 hour; take out the boiled carbon paper, mark it on the carbon paper, and take a 50mL small beaker Clean it up, and add an appropriate amount of ultrapure water to it, add the marked carbon paper to it, perform ultrasonic cleaning for 5-10 minutes, then replace the ultrapure water with ethanol and ultrasonically clean for 5 minutes; then put it in an oven for drying .
[0035] 1.2 Preparation of polyaniline nanoarrays (N-CNAs)
[0036] Take 50mL of ultr...
Embodiment 2
[0041] Example 2 Nitrogen-doped carbon nanoarray / cobalt ferrite material (N-C@CoFe 2 o 4 ) physicochemical properties.
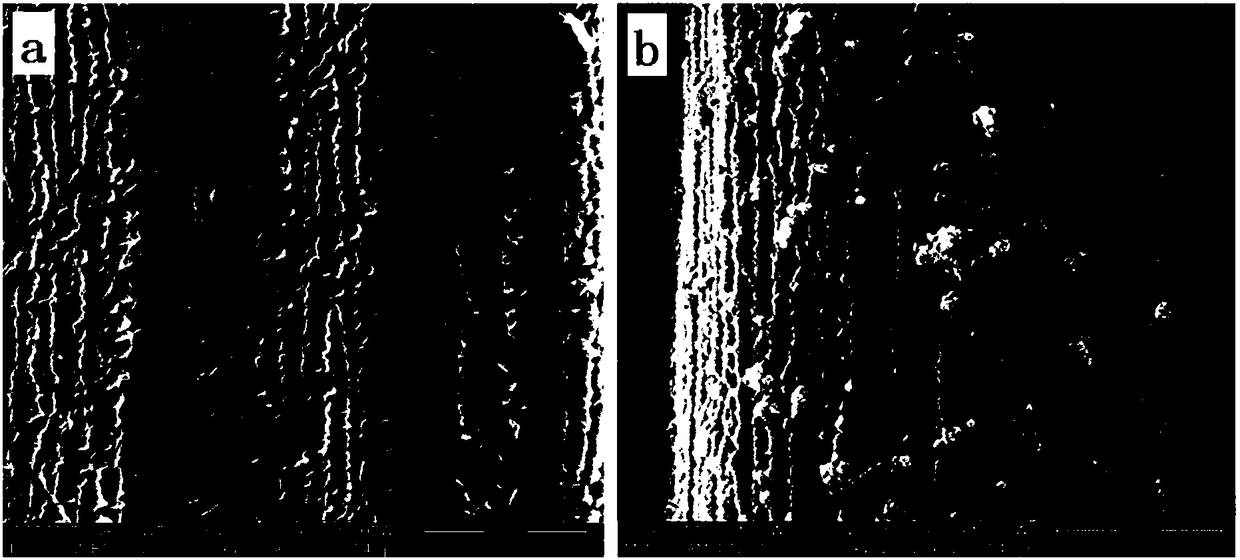
[0042] In embodiment 1, polyaniline nanoarray and figure 1 , a) SEM images of polyaniline nanoarrays (N-CNAs) during synthesis, b) nitrogen-doped carbon nanoarrays / cobalt ferrite (N-C@CoFe 2 o 4 ) of the scanning electron microscope, it can be seen that N-CNAs and N-C@CoFe were successfully prepared 2 o 4 .
Embodiment 3
[0043] Example 3 Nitrogen-doped carbon nanoarray / cobalt ferrite material (N-C@CoFe 2 o 4 ) catalytic activity.
[0044] 3.1 Linear sweep voltammetry
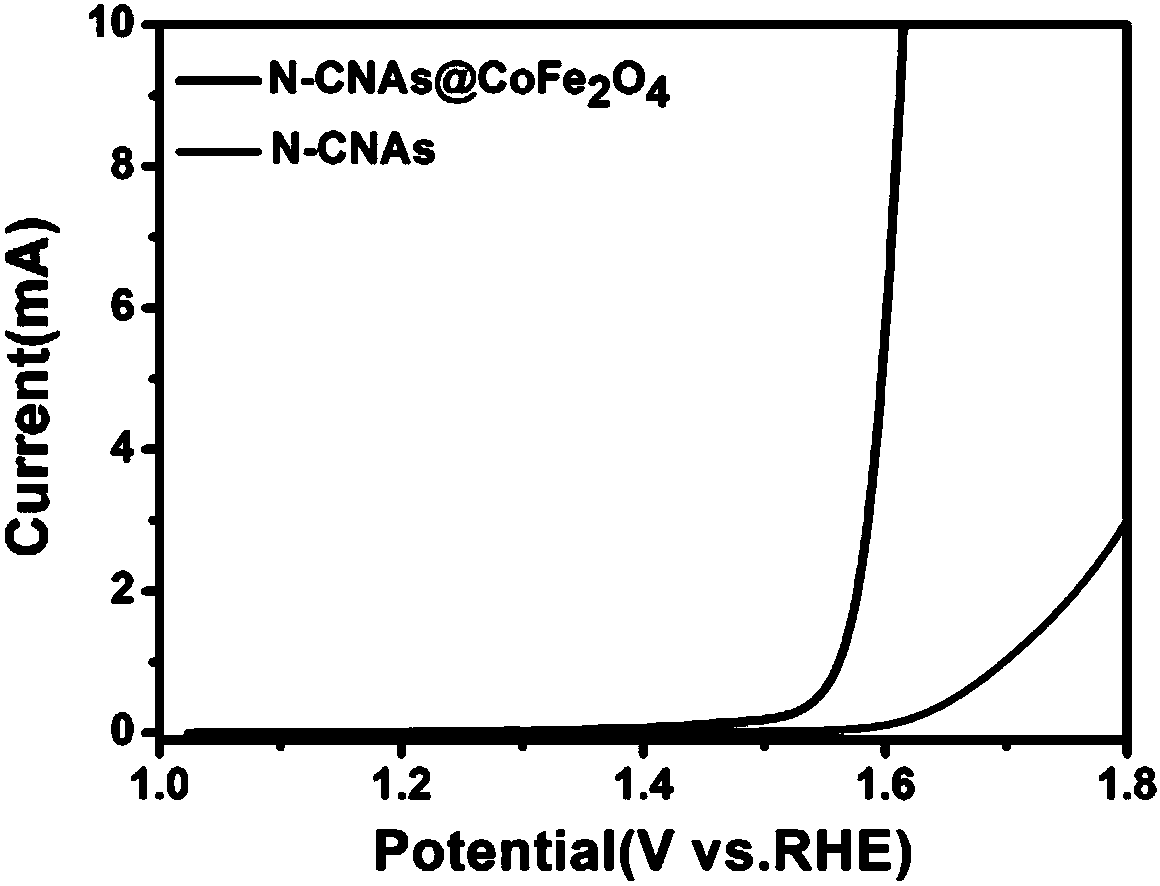
[0045] Detection of (0V~0.8V vs. Ag / AgCl) N-CNAs and N-C@CoFe in 1M KOH Electrolyte Solution 2 o 4 The corresponding linear sweep voltammetry curve is as figure 2 Shown: N-C@CoFe 2 o 4 The initial overpotential of N-CNAs is 1.42V, and that of N-CNAs is 1.57V. N-C@CoFe 2 o4 The initial overpotential is relatively low, and the potential required for oxygen evolution reaction is relatively low. It can be seen that N-C@CoFe 2 o 4 The OER catalytic activity of N-CNAs is stronger than that of N-CNAs.
[0046] 3.2 Electrochemical surface active area
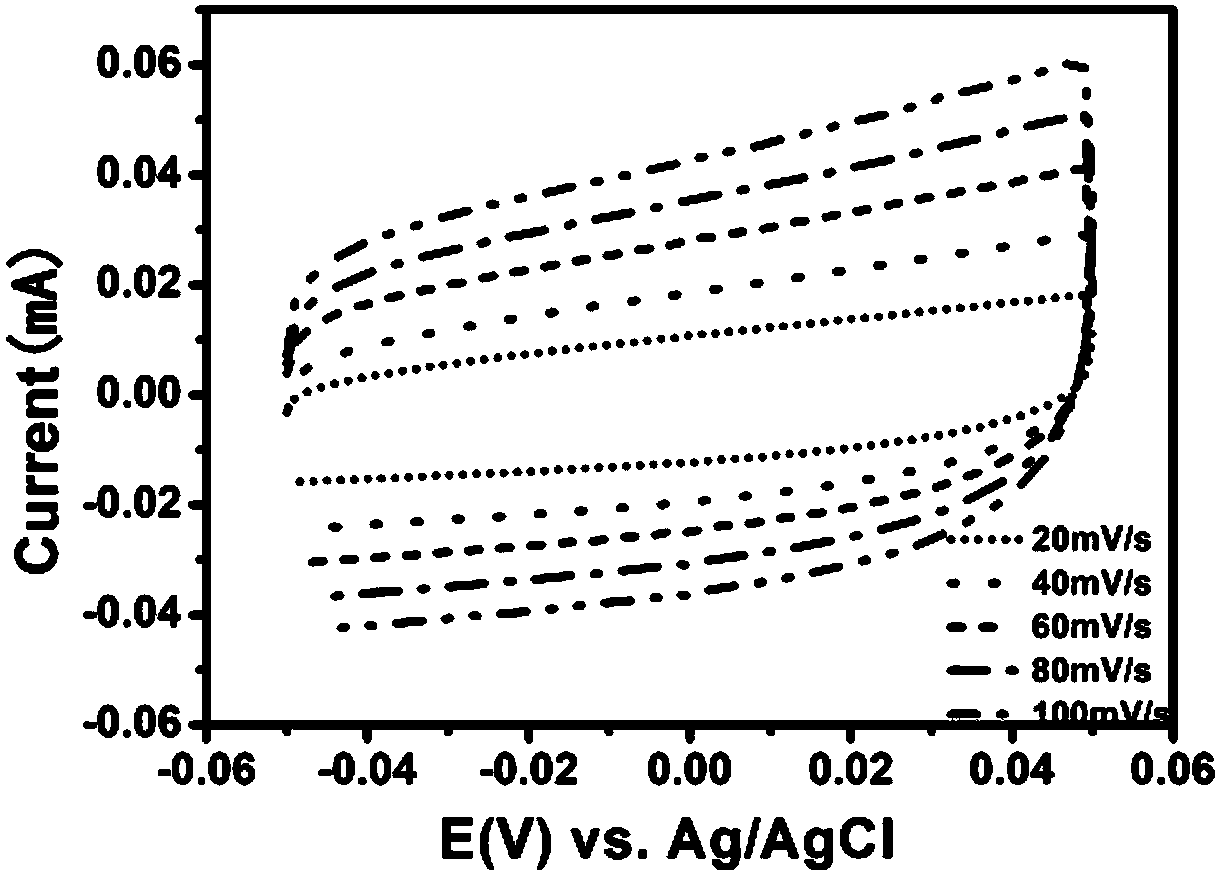
[0047] N-C@CoFe 2 o 4 The cyclic voltammetry curve (0V~0.8V vs.Ag / AgCl) tested in 1M KOH electrolyte solution is as follows image 3 As shown, N-C@CoFe was detected in 1M KOH electrolyte solution (-0.05V ~ 0.05V vs. Ag / AgCl, scan rate was 20, 40, 60, 80, 100mV / s) 2 o 4 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com