Albumin-stabilized MnO2 nanomaterial as well as preparation method and application
A technology of manganese dioxide and nanomaterials, applied in the field of functional biomimetic nano-drug delivery system, can solve the problems of time-consuming synthesis process, harsh reaction conditions, cumbersome water phase modification steps, etc., and achieve the effect of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
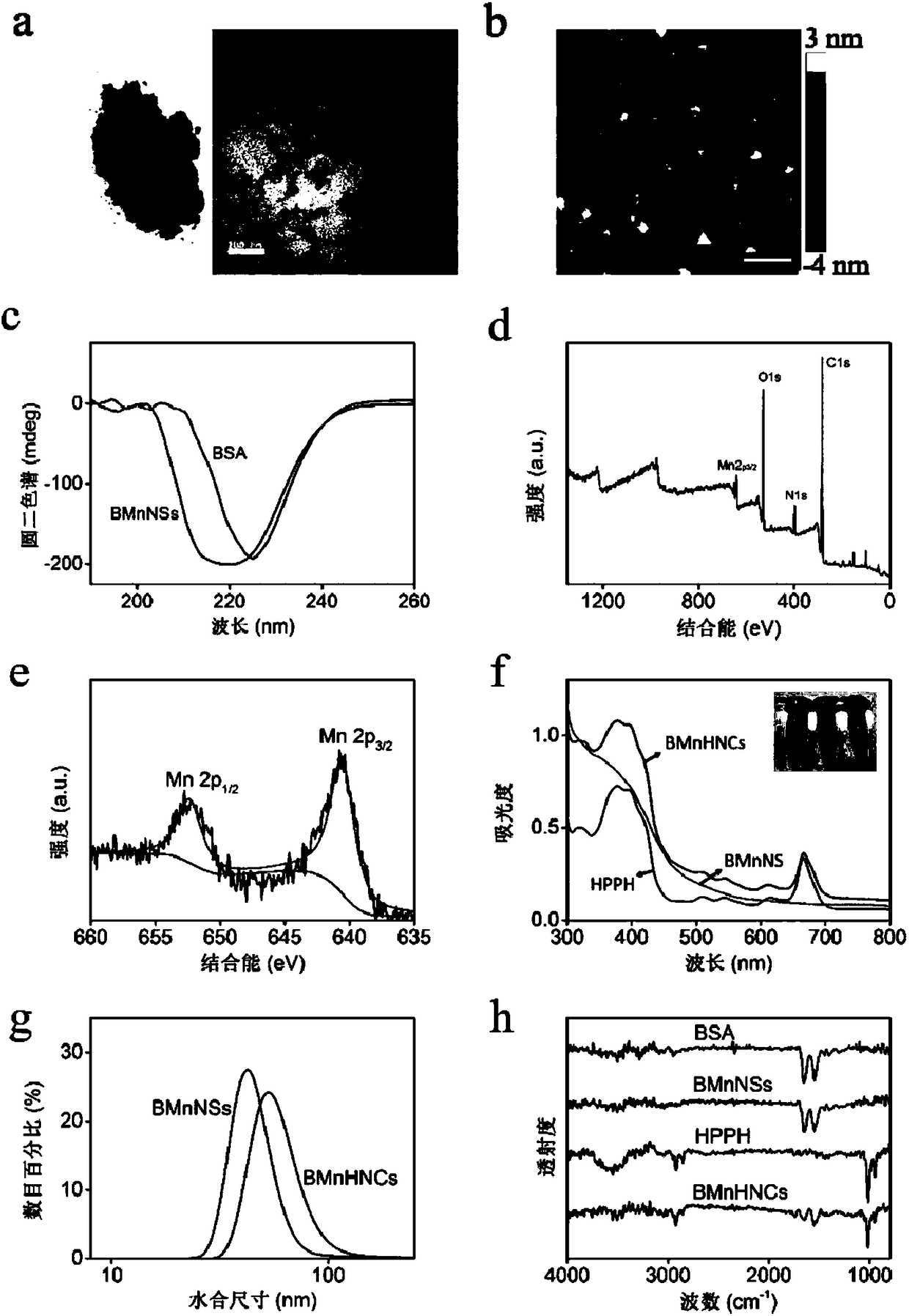
[0031] 1.BSA-MnO 2 Preparation of nanosheets (BMnNSs)
[0032] BMnNSs were synthesized by a biomineralization method using BSA as a stabilizer and reactive scaffold under physiological temperature conditions. At 37°C, 100 mM, 500 μL of MnCl 2 4H 2 O was quickly added to 10 mL of 10 mg / mL BSA solution, and the vortex mixer was shaken vigorously. After 3 minutes, quickly add 250 μL of 1M NaOH solution to adjust the pH to around 12. After adding NaOH, the cloudy solution immediately turned into a transparent light yellow color, and then, as the reaction progressed, the solution gradually turned dark brown. Stand at 37°C for 6 hours to promote crystal growth. Use MWCO=30KDa ultrafiltration tube ultrafiltration 4-5 times to terminate the reaction, 3250 rpm, 15 minutes, to remove excess reactants. Filtered with a 0.22 μm filter membrane, the obtained BMnNSs (BSA-MnO 2 Nanosheets) solution was dispersed in ultrapure water and stored in a 4°C refrigerator for later use.
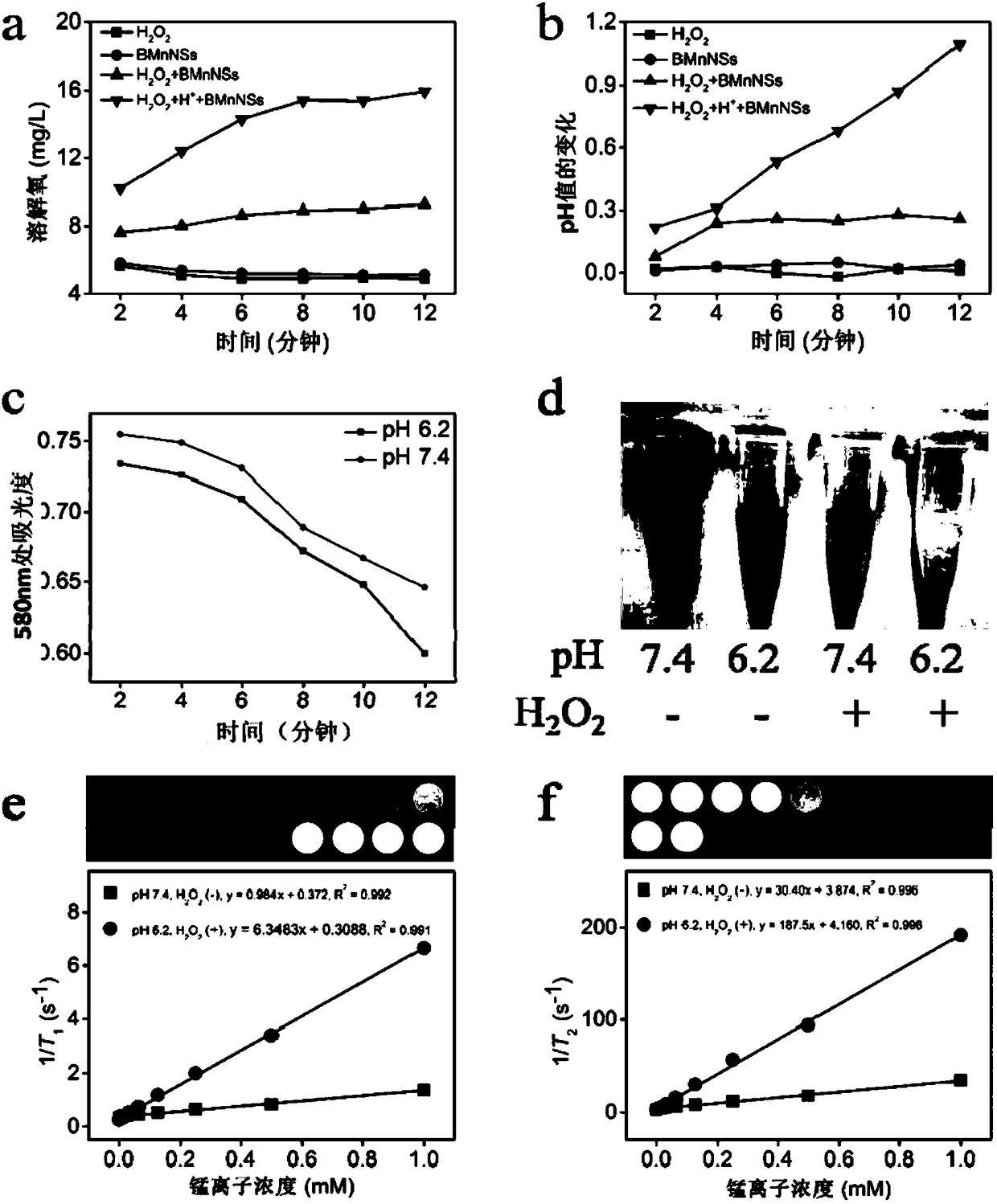
[003...
Embodiment 2
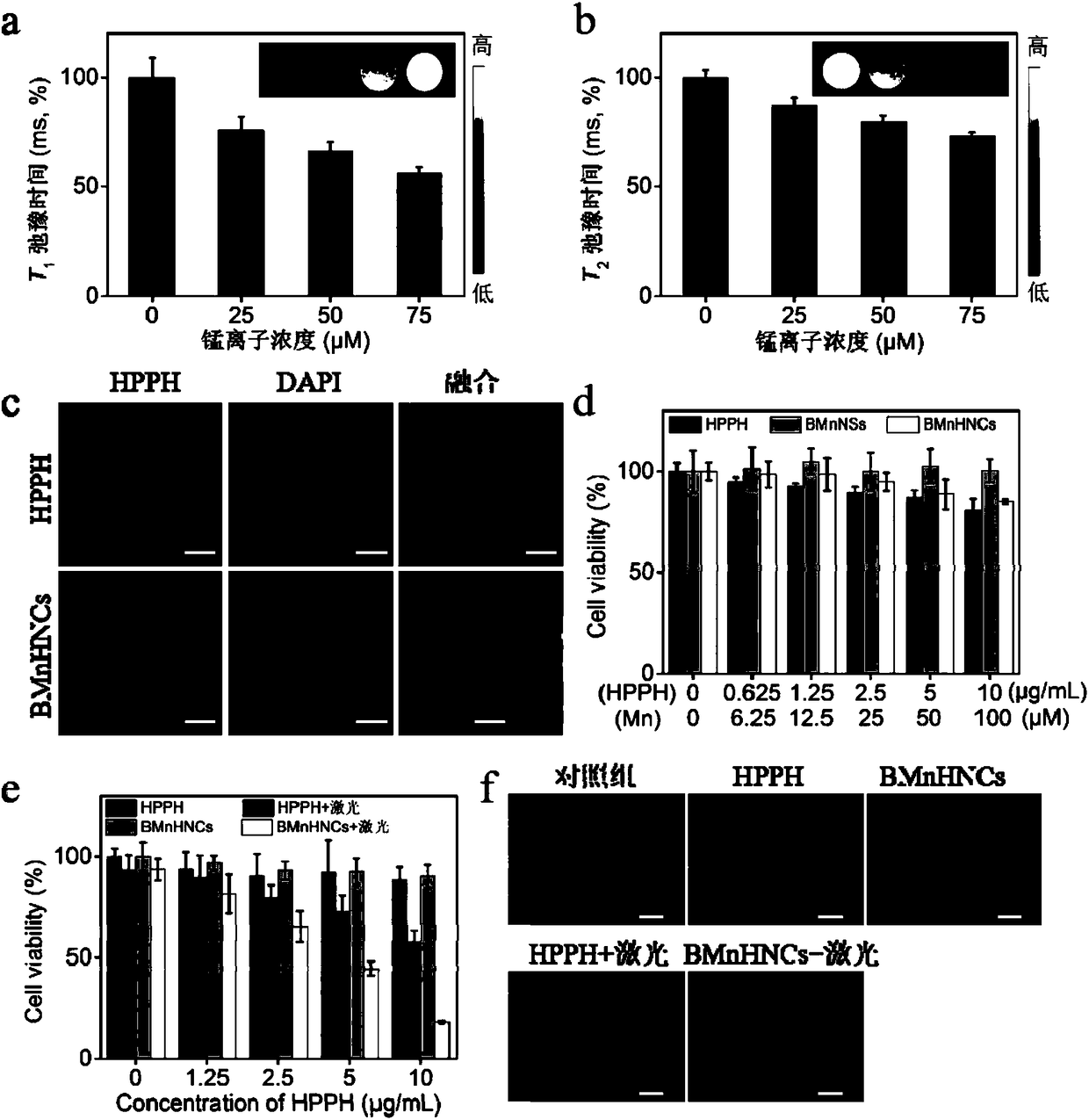
[0044] Example 2 In vitro cell experiment
[0045] Human glioblastoma cells (U87MG) were provided by Shanghai Academy of Sciences, China. The cells were cultured in DMEM medium containing 10% (v / v) serum, 1% penicillin and streptomycin, and placed in a 5% carbon dioxide incubator at 37°C.
[0046] In vitro cell fluorescence imaging: U87MG cells were incubated with HPPH or BMnHNCs (equivalent to 2 μg / mL HPPH) in the dark for 6 hours, then washed with PBS buffer three times, stained with DAPI, and confocal laser microscopy (CLSM, Olympus FV1200, Japan) to observe and take pictures.
[0047] Cell MRI imaging: U87MG cells were seeded in a 6cm cell culture dish, and when the cells were confluent to about 85%, different concentrations of BMnHNCs were added and incubated for 6 hours. The cells were washed three times with PBS buffer, digested, centrifuged, and collected. The cells were dispersed in 200 μL of 1% low-melting point agarose, and the in vitro cell MRI imaging was perfo...
Embodiment 3
[0054] After the cells were incubated with HPPH or BMnHNCs and irradiated with 630nm laser, the viability of the cells was significantly lower than that of the group without laser irradiation ( image 3 e). After laser irradiation, the viability of BMnHNCs-treated cells was lower than that of HPPH-treated cells. This could be explained by increased cellular internalization efficiency, which was confirmed by cellular CLSM results. Since singlet oxygen is involved in the photodynamic therapy-mediated cell killing effect, from MnO 2 and intracellular H 2 o 2 The O produced by the reaction 2 It can improve the effect of photodynamic therapy. Subsequently, the applicant used the calcein-acetyl hydroxymethyl ester staining method to observe the photodynamic killing effect of HPPH or BMnHNCs on U87MG cells. Such as image 3 As shown in f, U87MG cells treated with only HPPH or only BMnHNCs showed the same green fluorescence as the control cells. However, the cells treated with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com