Niraparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
A technology for controlled release drugs and compositions, which is applied in the field of PARP enzyme inhibitor drugs and niraparib sustained and controlled release pharmaceutical compositions, and can solve problems that have nothing to do with research on oral sustained and controlled release preparations of niraparib.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
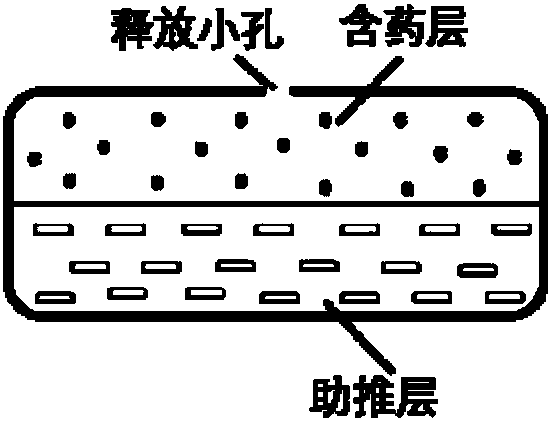
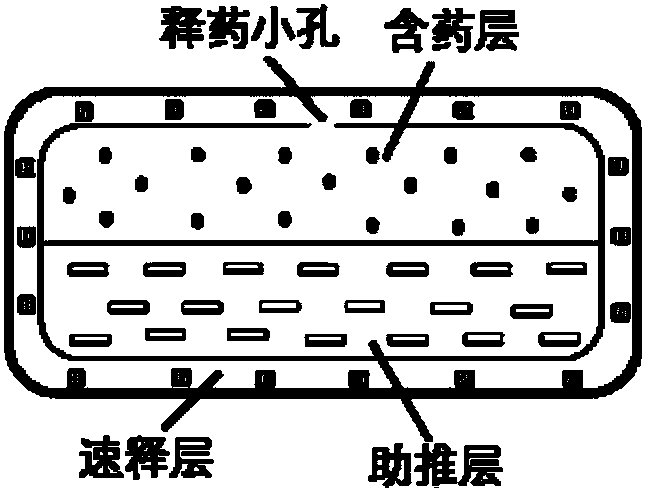
[0086] The preparation method of Niraparib osmotic pump controlled-release tablet comprises the following steps: 1. preparation of Niraparib in improved dissolution form; 2. preparation of drug-containing layer; 3. preparation of push layer; 4. preparation of double-layer tablet; Preparation of the selected double-layer isolation coating; ⑥ Preparation of the controlled-release coating; ⑦ Perforation of the controlled-release coating of the osmotic pump tablet; ⑧ An optional aesthetic coating layer; ⑨ An optional immediate-release drug-containing layer. The above ②-⑨ can be carried out by conventional pressing and coating methods well known to those skilled in the art.
[0087] The tablet with the immediate-release drug-containing layer outside the rigid membrane shell is an osmotic pump rapid-sustained double-release tablet, while the tablet without an immediate-release drug-containing layer outside the rigid membrane shell is an ordinary osmotic pump controlled-release tablet...
specific Embodiment
[0126] The following examples generally describe the preparation methods and / or characterization results of typical compositions of the present invention, and all percentages are by weight unless otherwise indicated. The following examples are specific illustrations of the present invention, but should not be considered as limiting the scope of the present invention. In the following examples, various procedures and methods not described in detail are conventional methods well known in the art.
[0127] Experimental animals: 6 Beagle dogs, half male and half male, weighing 8-10 kg. The sources are all from Beijing Masi Biotechnology Co., Ltd. The test animals were adaptively fed in the experimental site of the Experimental Animal Center of Shanghai Institute of Materia Medica 14 days before the test day.
[0128] Using a single-punch tablet press (TDP-1, Guangzhou Xulang Machinery Equipment Co., Ltd.)
Embodiment 1
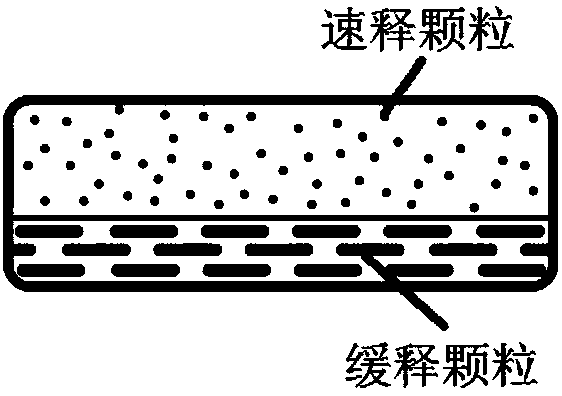
[0129] Example 1 Fast and slow double-effect release matrix tablet (200 tablets)
[0130]
[0131] Immediate-release layer: After the prescription amount of Niraparib is mixed with the solubilizing matrix excipient Soluplus and micropowder silica gel, it is prepared into a solid dispersion by melt extrusion, crushed, and passed through a 60-mesh sieve. Agent crospovidone PVPP XL and lubricant magnesium stearate are mixed evenly and ready for tableting;
[0132] Sustained-release layer: Niraparib of prescription quantity and solubilization matrix excipient copovidone (PVP VA64) and micropowder silica gel are prepared into solid dispersion with the above-mentioned melt extrusion method, and then with the release rate adjustment of prescription quantity with slow release layer Release matrix material HPMC K15M (BASF, Germany) and lubricant magnesium stearate, mix well, and wait for tabletting.
[0133] Tablet compression: the direct compression method is used to make matrix t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com