Oxidization-state mercury selective adsorbent and preparation method
A technology of oxidized mercury and adsorbents, applied in chemical instruments and methods, separation methods, alkali metal oxides/hydroxides, etc., to achieve small artificial introduction errors, good sulfur and water resistance, and simple post-processing and analysis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 16.4g Ca(NO 3 ) 2 and 6g of mesoporous SiO 2 The powder was added to 100ml of deionized water, and stirred with a magnetic stirrer at room temperature for 6 hours, and then the mixed solution was placed in an oven at 105°C for 12 hours, and completely dried; the dried sample was placed in a nitrogen atmosphere at Calcined at 750°C for 4 hours to obtain massive solids; grind the solids on a mortar and pass through a 18-24 mesh sieve to obtain the corresponding solid particles, which are adsorbent 1, and store the adsorbent 1 in silica gel spare.
[0035] Weigh 16.4g Ca(NO 3 ) 2 , 7.45g KCl and 6g mesoporous SiO 2 The powder was added to 100ml of deionized water, and stirred with a magnetic stirrer at room temperature for 6 hours, and then the mixed solution was placed in an oven at 105°C for 12 hours, and completely dried; the dried sample was placed in a nitrogen atmosphere at Calcined at 750°C for 4 hours to obtain blocky solids; grind the solids on a morta...
Embodiment 2
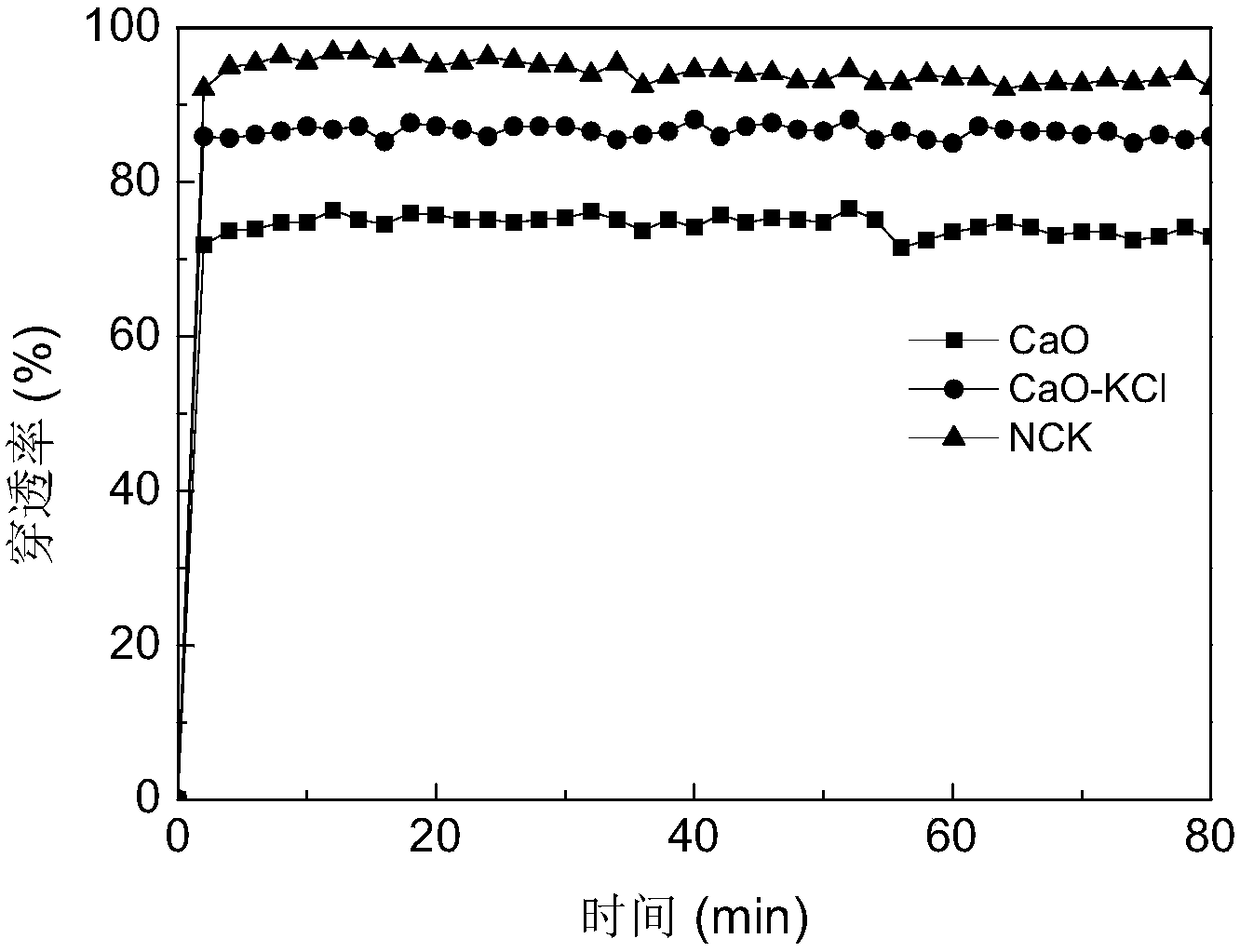
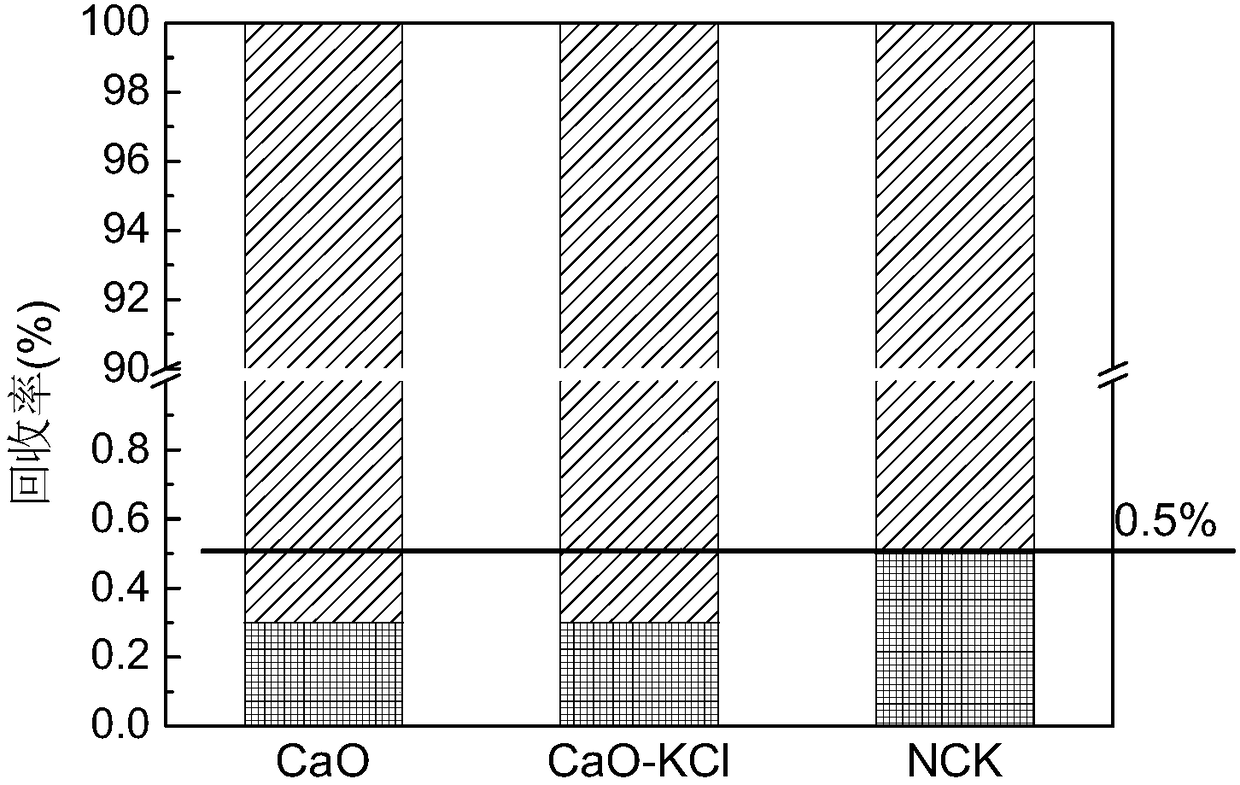
[0040] Adsorbents 3, 4, and 5 in Example 1 were selected to verify their mercury adsorption performance on a fixed-bed adsorption test bench. The fixed bed adsorption device consists of five parts: simulated flue gas generation system, mercury vapor generation system, quartz tube reactor, mercury measurement system and tail gas treatment device. Hg of the adsorbent 0 The adsorption performance is evaluated, the elemental mercury source produces constant mercury vapor, which is brought into the reactor by high-purity nitrogen, and the inlet mercury concentration is 34±2μg / m 3 . The inner diameter of the quartz tube reactor is 8 mm, the amount of adsorbent used is 300 mg, and the bed height is about 8 mm. The adsorption temperature is 120°C, the total gas volume is 2L / min, the gas component is pure nitrogen, and the adsorption time is 80min. The adsorption performance of elemental mercury is determined by Hg 0 The penetration rate is defined as the ratio of the mercury conce...
Embodiment 3
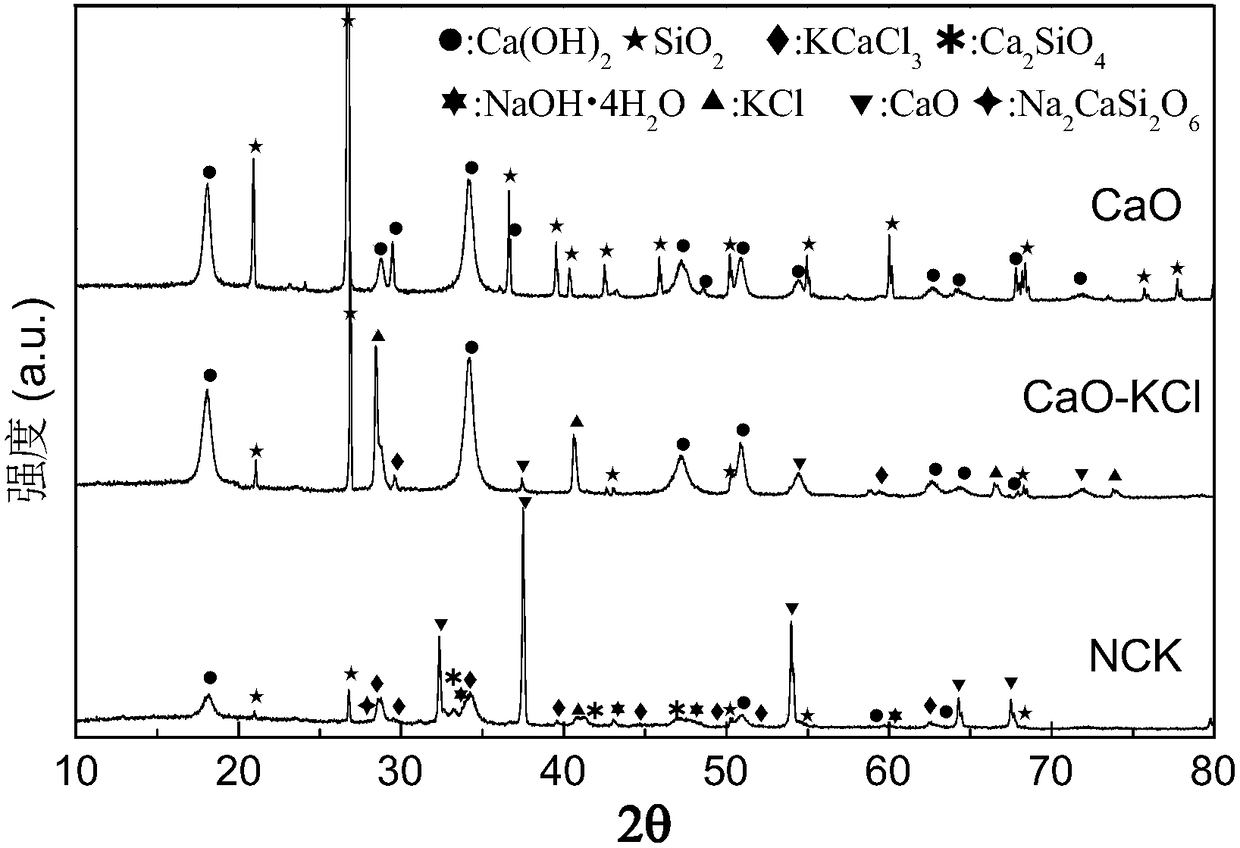
[0043] Adsorbents 1, 2, and 4 in Example 1 were treated with a certain amount of water vapor under ambient conditions, and the crystal structures of the three adsorbents were analyzed using an ARL X'TRA type X-ray diffractometer (Thermo, U.S.). Determination, experimental condition parameters: Cu target (Kα ray, ), Ni filter, the tube voltage is 40kV, the current through the tube is 30mA, scan at a speed of 0.1° / S in the range of 10-80° and use Jade 6.0 software to fit the spectrum to obtain an accurate crystal structure .
[0044] exist figure 1 It can be clearly observed in the XRD characterization results of the three adsorbents that CaO and Ca(OH) 2 Diffraction peaks, under the same treatment conditions, the oxidized mercury selective adsorbent of the present invention retains more CaO, while there are more Ca(OH) in the other two adsorbents 2 , indicating that water vapor interacted with CaO in the two adsorbents. At the same time, it also shows that the adsorbent is...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap