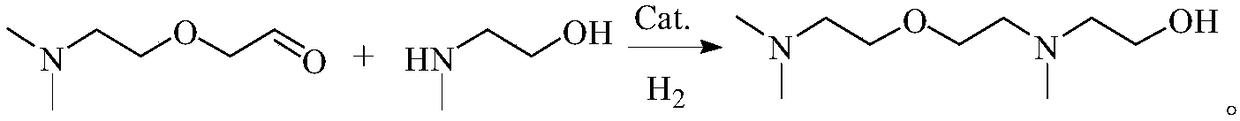Preparation method of N,N,N'-trimethyl-N'-hydroxyethyl diaminoethyl ether
A technology of bisaminoethyl ether and dimethylamino, which is applied in the field of polyurethane industry, can solve the problems of low atom economy, little application value, and complicated processing, and achieve high atom economy, low pollution, and low raw material cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
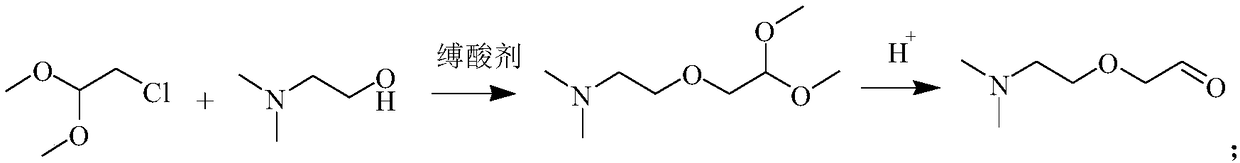
[0047] Embodiment 1, a kind of preparation method of 2-[2-(dimethylamino) ethoxyl] acetaldehyde, take chloroacetaldehyde dimethyl acetal and N, N-dimethylethanolamine as raw material, after adding first hydrolysis:
[0048] Add 12.4g (0.1mol) of chloroacetaldehyde dimethyl acetal, 8.9g (0.1mol) of N,N-dimethylethanolamine, 4.0g (0.1mol) of sodium hydroxide and 30ml of solvent toluene into a 250ml three-neck flask. Heat to reflux for 8 hours, cool down to room temperature after the reaction, and analyze the still liquid by gas chromatography. At this time, the conversion rate of the raw material (ie, N,N-dimethylethanolamine) is 91.8%, and the product yield is 88.3%.
[0049]Keep stirring at room temperature and slowly add concentrated hydrochloric acid (concentration: 37%) dropwise (control the temperature of the system not to exceed 50° C. during the dropwise addition) until pH=1. After the dripping, the layers were left to stand, and the water layer was taken for the next s...
Embodiment 1-1~ Embodiment 1-7
[0051] Change the reaction condition of nucleophilic substitution among the embodiment 1, promptly change the raw material mol ratio (N in the embodiment 1, N-dimethylethanolamine: chloroacetaldehyde dimethyl acetal, the consumption of N, N-dimethylethanolamine keeps constant), sodium hydroxide consumption, reflux time, solvent name (solvent consumption remains constant), and the resulting total yield is shown in Table 1.
[0052] Table 1
[0053] Example
1
1-1
1-2
1-3
1-4
1-5
1-6
1-7
Reaction time / h
8
6
7
8
9
10
8
8
Sodium hydroxide dosage / g
4.0
4.0
4.0
4.4
4.0
4.0
4.4
4.4
Raw material molar ratio
1
1.1
1.1
1.1
1.1
1.1
1.3
1.3
solvent
toluene
toluene
toluene
toluene
water
Raw material conversion rate / %
91.8
62.3
75.7
92.3
92.0
92.0
91.8
15.0
Product ...
Embodiment 2
[0066] Example 2, 2-[2-(dimethylamino)ethoxy]acetaldehyde and N-methylethanolamine hydroamination to prepare N,N,N'-trimethyl-N'-hydroxyethyl Aminoethyl ether:
[0067] 13.1 g (0.1 mol) of 2-[2-(dimethylamino)ethoxy]acetaldehyde, 8.3 g (0.1 mol) of N-methylethanolamine, 1.3 g of Raney Ni and 15 ml of methanol were put into a 100 ml autoclave. At room temperature (about 20°C), fill the kettle with hydrogen to 2.0MPa, heat to 120°C and stir for 8h, keeping the pressure inside the kettle (adjusted by hydrogen) at 2.0-2.2MPa.
[0068] After the reaction was completed, the temperature was cooled to room temperature and filtered. The obtained filter cake is immediately put into a concentrated sodium hydroxide solution with a mass concentration of 40% and soaked for at least 30 minutes, and then washed with clear water for several times (until the pH of the washing solution is about 8), and then Raney Ni can be recycled. The filtrate was analyzed by gas chromatography, and the conv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com