Allyl Alcohol Oxidation Method
An allyl alcohol and oxidant technology, applied in chemical instruments and methods, organic chemistry, molecular sieve catalysts, etc., can solve the problems of non-renewable, large environmental pollution, difficult application, etc. Effect of reproduction frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0091] According to the method of the present invention, the amounts (mass) of the titanium-silicon molecular sieves loaded in the first to nth catalyst beds may be the same or different. According to one implementation, when m is any integer in the interval [2, n], W m-1 / W m 0.1-20, W m-1 / W m Preferably it is 0.5 or more, More preferably, it is 1 or more, More preferably, it is 2 or more. Here, W m-1 is the quality of the catalyst packed in the upstream catalyst bed in any pair of adjacent catalyst beds from the first catalyst bed to the last nth catalyst bed, W m is the mass of the catalyst loaded in the downstream catalyst bed in any pair of adjacent catalyst beds from the first catalyst bed to the nth catalyst bed. W m-1 / W m Preferably it is 15 or less, and more preferably 10 or less. More preferably, W m-1 / W m for 2-8. W m-1 and W m It is determined by the content of the titanium-silicon molecular sieve in the shaped titanium-silicon molecular sieve. In ...
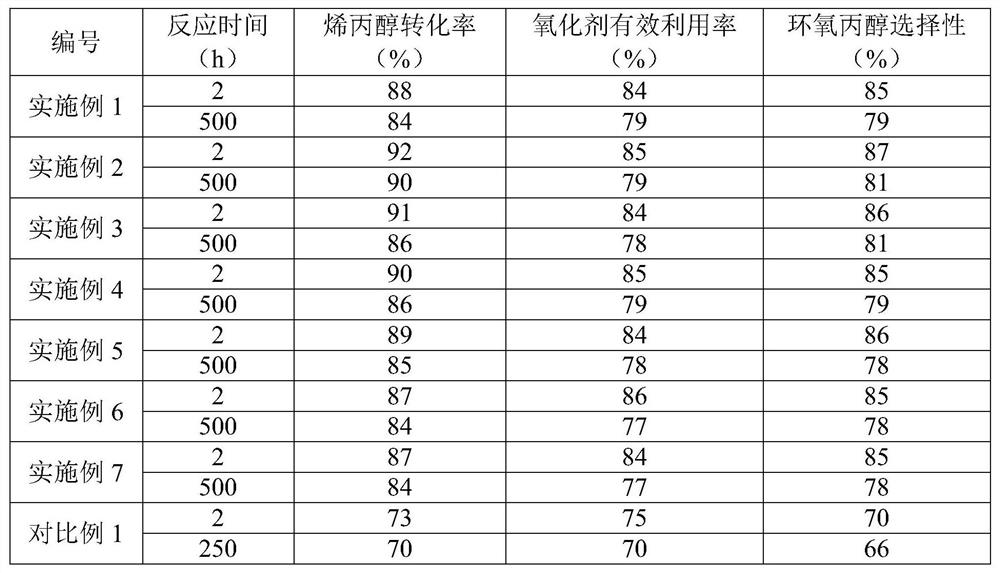
Embodiment 1
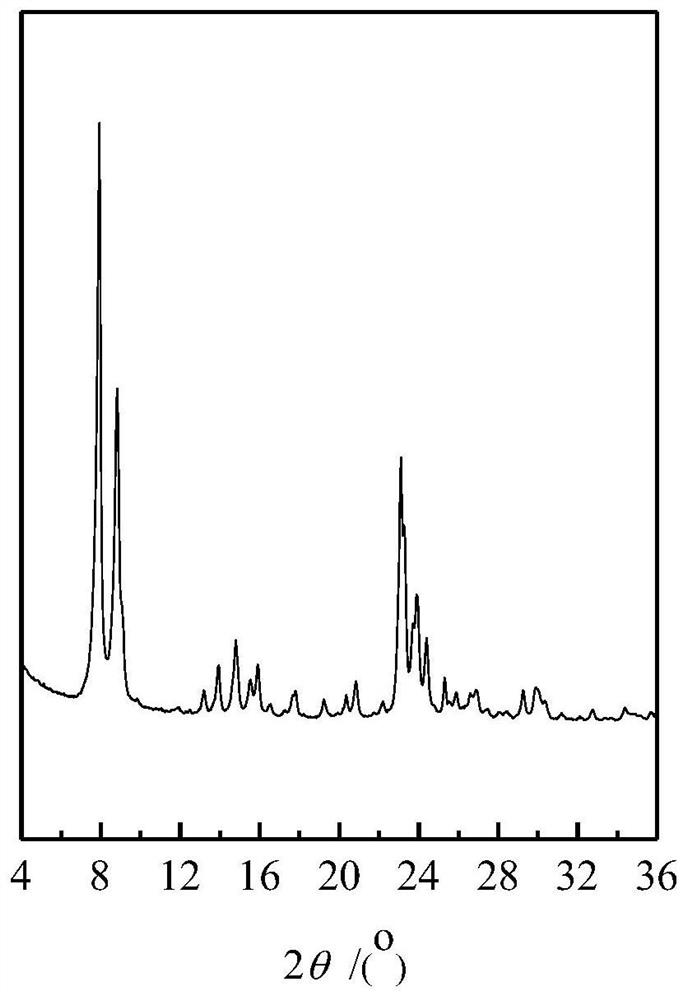
[0120] The catalyst used in this example is modified titanium-silicon molecular sieve TS-1, and the titanium-silicon molecular sieve TS-1 is prepared with reference to the method described in Zeolites, 1992, Vol.12 pages 943-950. The specific method is: At 20°C, mix 22.5g tetraethyl orthosilicate with 7.0g tetrapropylammonium hydroxide as a template, add 59.8g distilled water, stir and mix, then hydrolyze at normal pressure and 60°C for 1.0h to obtain Hydrolyzed solution of tetraethylorthosilicate. Under vigorous stirring, a solution consisting of 1.1 g of tetrabutyl titanate and 5.0 g of anhydrous isopropanol was slowly added to the hydrolysis solution, and the resulting mixture was stirred at 75° C. for 3 h to obtain a clear transparent colloid. The colloid was placed in a sealed stainless steel reaction kettle, and kept at a constant temperature of 170° C. for 36 hours to obtain a mixture of crystallized products. The obtained mixture was filtered, the collected solid matt...
Embodiment 2
[0127] The catalyst used in this example is modified titanium-silicon molecular sieve TS-1, and the titanium-silicon molecular sieve TS-1 before modification is prepared by the following method.
[0128] Dissolve tetrabutyl titanate in the alkali source template agent tetrapropyl ammonium hydroxide aqueous solution first, then add silica gel (purchased from Qingdao Silica Gel Factory) to obtain a dispersion. In the dispersion, silicon source: titanium source: alkali source Template agent: water molar ratio is 100:4:12:400, silicon source is SiO 2 In terms of titanium source as TiO 2 In terms of alkali source template agent in N. Seal the above dispersion in the beaker with a parafilm and let it stand at room temperature (25°C, the same below) for 24h, then stir at 35°C for 2h with magnetic stirring to redisperse it. Transfer the re-dispersed dispersion liquid to a sealed reaction kettle, undergo the first stage of crystallization at 140°C for 6h, then cool the mixture down t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com