Method for determining contents of pentose, hexose, amino sugar and uronic acid in pneumonic polysaccharide vaccine hydrolysate
A determination method and hydrolyzate technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of simultaneous detection of multiple monosaccharides, influence on quantitative accuracy, long measurement time, etc., and achieve unique separation selectivity and The effect of anti-matrix interference ability, long-term stability, rapid equilibrium, good retention and separation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 1. Experimental instruments and equipment:
[0072] High pressure binary pump, degasser, autosampler, column oven and triple quadrupole mass spectrometer.
[0073] 2. Experimental reagents:
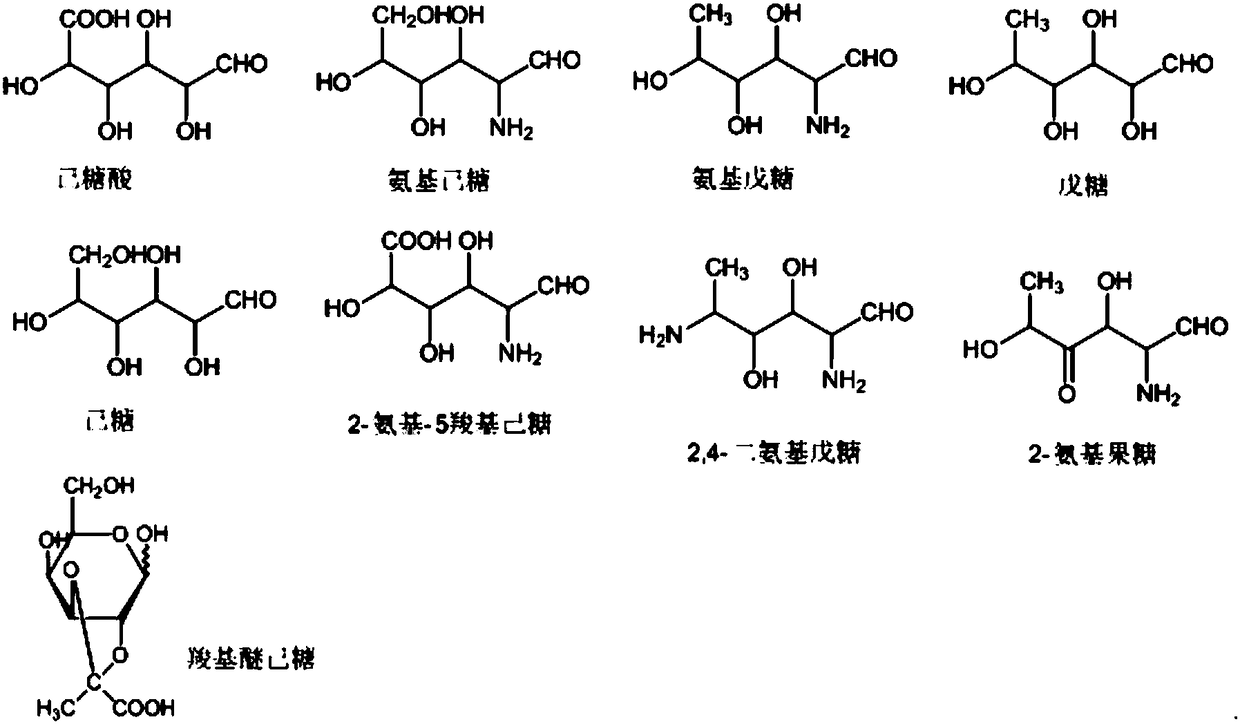
[0074] Glucose, rhamnose, glucuronic acid, and rhamnose amino were purchased from Sigma, and the specifications were all 1 g; Ether glucose was synthesized in the laboratory, and the structure of the synthesized compound was confirmed by MS and literature data.
[0075] 3. Detection conditions:
[0076] Chromatographic column: stationary phase 1, R1 is silica gel;
[0077] Mobile phase: A-acetonitrile aqueous solution containing 10mmol / L ammonium formate (adjust pH to 4.3 with formic acid), the volume concentration of acetonitrile in the acetonitrile aqueous solution is 80%; B-10mmol / L ammonium formate (adjust pH to 4.3 with formic acid) aqueous solution ;
[0078] Specifically, the final concentration of ammonium formate in mobile phase A in aqueous acetonitrile is 10 mmol / L....
Embodiment 2
[0099] Detection of 2,4-diaminopentose and hexonic acid in polysaccharides from type 1 pneumonia
[0100] Type 1 pneumonia polysaccharides can be hydrolyzed to obtain 2,4-diaminopentose and hexonic acid. The detection conditions are as follows:
[0101] Stationary phase: with stationary phase described in embodiment 1;
[0102] Mobile phase: A-acetonitrile aqueous solution containing 10mmol / L ammonium formate (adjust pH to 6.5 with acetic acid), the volume concentration of acetonitrile in the acetonitrile aqueous solution is 80%; B-10mmol / L ammonium formate (adjust pH to 6.5 with acetic acid) aqueous solution ;
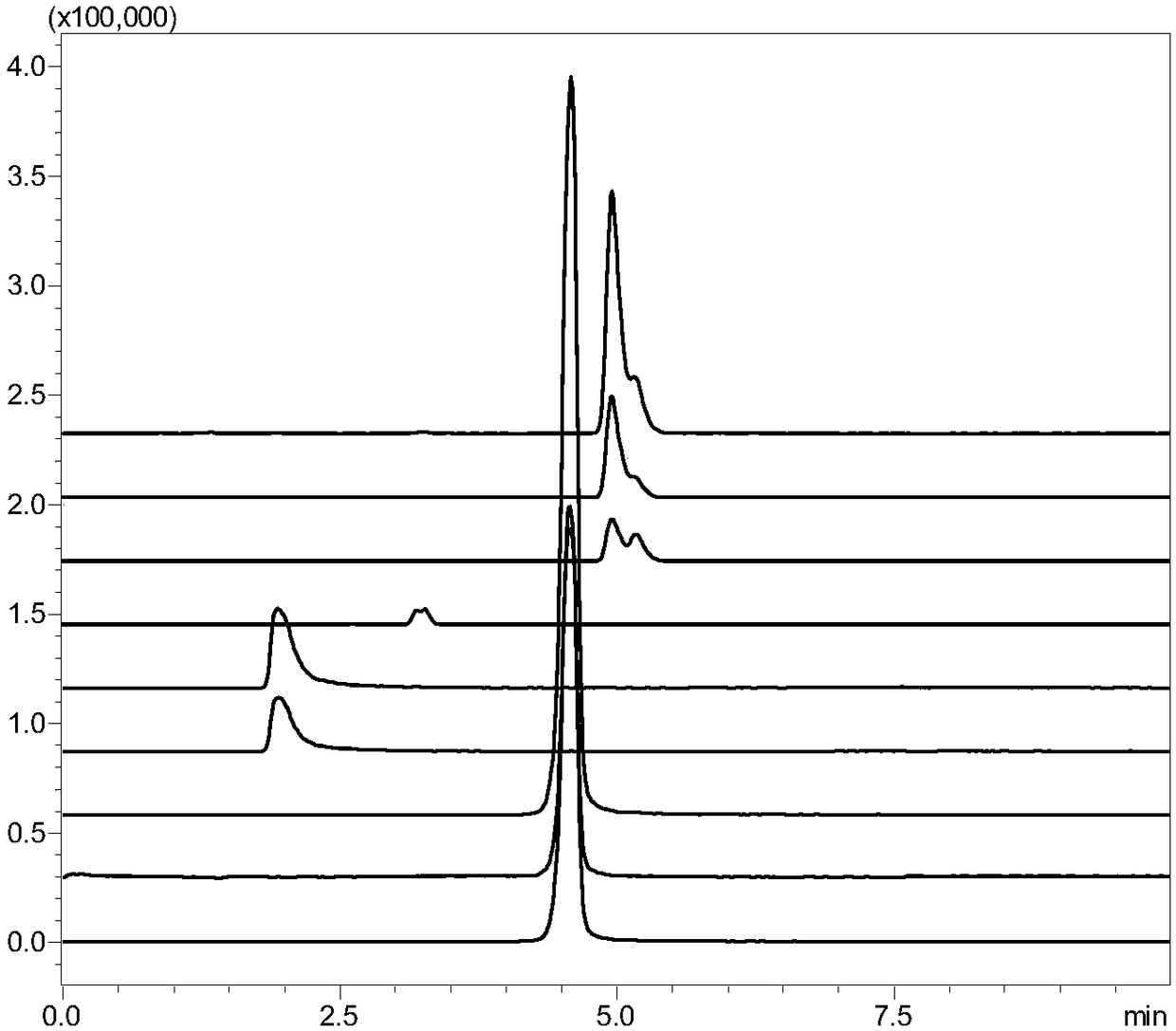
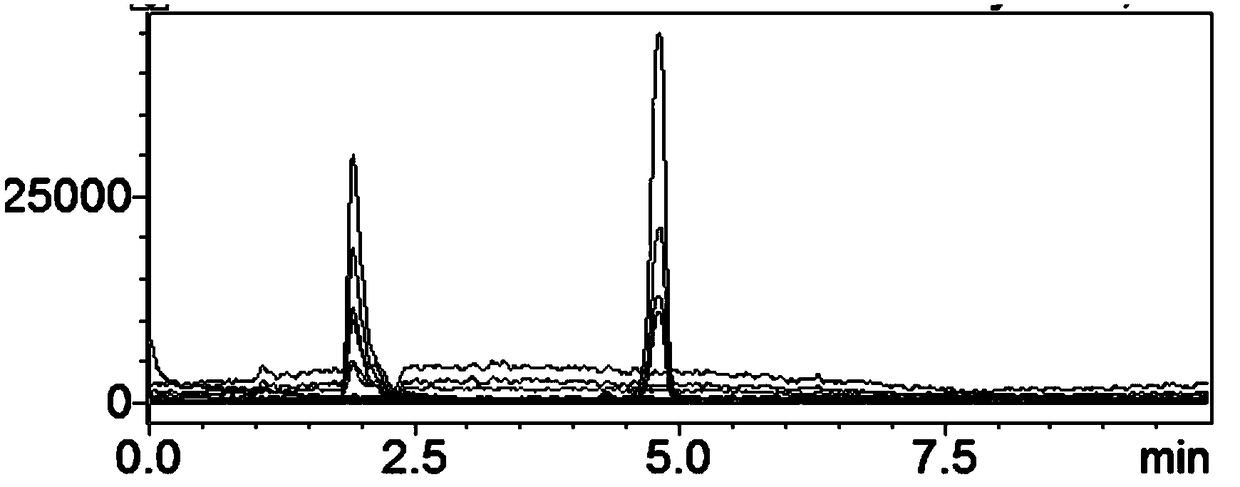
[0103] Gradient: 0-10min 80%A-20%A; LC-MS conditions: Ion source: ESI positive and negative ion scanning simultaneously, nebulizer flow rate 3L / min, heater flow rate 10L / min, interface temperature 200°C, DL temperature 230 °C, the temperature of the heating module is 400 °C, the drying gas flow rate is 10 L / min, and the interface voltage is 3 kV; other detection co...
Embodiment 3
[0105] Example 3: Detection of pentose, hexose and hexonic acid in type 2 pneumonia polysaccharide
[0106] Type 2 pneumonia polysaccharides can be hydrolyzed to obtain pentose sugar, hexose sugar and hexonic acid. The detection conditions are as follows:
[0107] Stationary phase: with stationary phase described in embodiment 1;
[0108] Mobile phase: A-contains 5mmol / L ammonium formate (pH 6.5) aqueous methanol solution, the volume concentration of methanol in the methanol aqueous solution is 80%; B-5mmol / L ammonium formate (pH 6.5) aqueous solution;
[0109] Gradient: 0-10min 75%A-20%A; LC-MS conditions: ion source: ESI negative ion, nebulizer flow rate 3L / min, heater flow rate 10L / min, interface temperature 200°C, DL temperature 230°C, heating The module temperature is 400° C., the drying gas flow rate is 10 L / min, and the interface voltage is 3 kV; other testing conditions are the same as those in Example 1.
[0110]The measured pentose, hexose and hexonic acid content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com