Oral film for treating infantile hemangioma and preparation method thereof
A technology of infantile hemangioma and oral film, which is applied in the field of pharmaceutical preparations, can solve problems such as adverse effects, hypoglycemia, and bradycardia, and achieve the effects of improving acceptance, accurate quantitative administration, and increasing adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, preliminary experiment
[0052] (1. Purpose
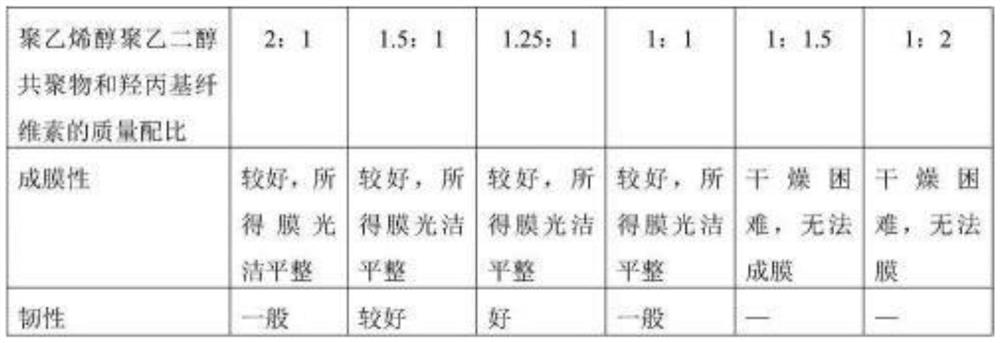
[0053] Investigate the effect of the film-forming material polyvinyl alcohol polyethylene glycol copolymer (BASF company, pharmaceutical excipient approval number F20110024) and hydroxypropyl cellulose on the film-forming properties
[0054] (2) Methods and results
[0055] According to the water-soluble characteristics of hydroxypropyl cellulose, and considering that hydroxypropyl cellulose (HPC) is a temperature-sensitive cellulose derivative, too high a temperature will destroy the structure of hydroxypropyl cellulose or even To make it lose its viscosity, add water to swell and dissolve it, and prepare it with a mass concentration of 2.5%, 5%, 7.5%, and 10%, respectively, and the temperature increases (60°C, 70°C, 80°C, 90°C) when dissolving A series of hydroxypropyl cellulose film-forming solutions were spread on petri dishes by homogenate casting film-forming method, and dried naturally, and the appeara...
Embodiment 2
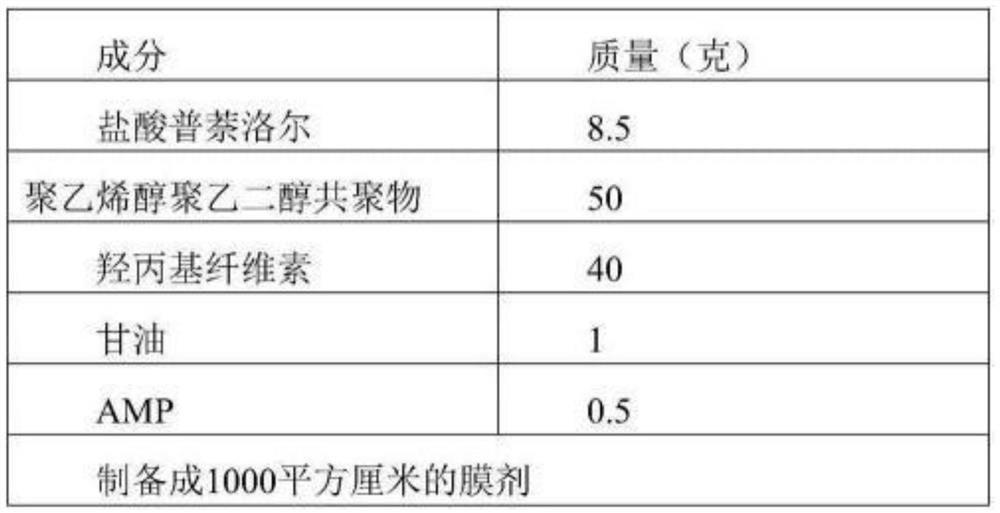
[0069] Embodiment 2, prescription and preparation of propranolol hydrochloride oral film
[0070]Table 2 Prescription:
[0071]
[0072] preparation:
[0073] (1) Add the film-forming material into purified water, heat to 70°C, and stir to dissolve;
[0074] (2) Add propranolol hydrochloride, plasticizer and flavoring agent into purified water, heat to 70°C, and stir to dissolve;
[0075] (3) Add the mixture obtained in step (1) into the mixture obtained in step (2), and stir for 10 min;
[0076] (4) removing the air bubbles in the mixed liquid obtained in step (3);
[0077] (5) The mixed liquid obtained in step (4) is lowered to room temperature, the coating thickness is set to be 60-180 μm, and the film is coated with a film coating machine;
[0078] (6) The film obtained in the step (5) was dried at 50° C. until the water content was 3% by mass, and the film was peeled off, dosed, and packaged sequentially, so as to obtain the oral film of propranolol hydrochloride. ...
Embodiment 3
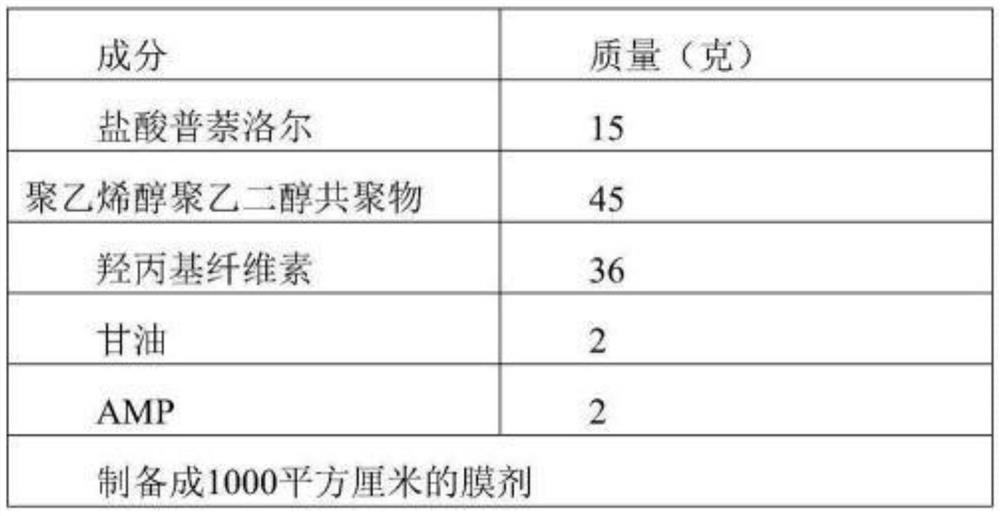
[0079] Embodiment 3, prescription and preparation of propranolol hydrochloride oral film
[0080] Table 3 Prescription:
[0081]
[0082] preparation:
[0083] (1) Mix the two components of the film-forming material, add to purified water, heat to 75°C, and stir to dissolve;
[0084] (2) Add propranolol hydrochloride, plasticizer and flavoring agent into purified water, heat to 75°C, and stir to dissolve;
[0085] (3) Add the mixture obtained in step (1) into the mixture obtained in step (2), and stir for 8 minutes;
[0086] (4) removing the air bubbles in the mixed liquid obtained in step (3);
[0087] (5) The mixed liquid obtained in step (4) is lowered to room temperature, the coating thickness is set to be 60-180 μm, and the film is coated with a film coating machine;
[0088] (6) The film obtained in the step (5) was dried at 55° C. until the water content was 6% by mass, and the film was peeled off, dosed, and packaged sequentially, so as to obtain the oral film o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com