Method for compounding drug intermediate allylthiourea
A technology of propylene-based thiourea and a synthesis method, applied in directions such as organic chemistry, can solve problems such as low final yield and complicated process, and achieve the effects of shortening reaction time, reducing intermediate links and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
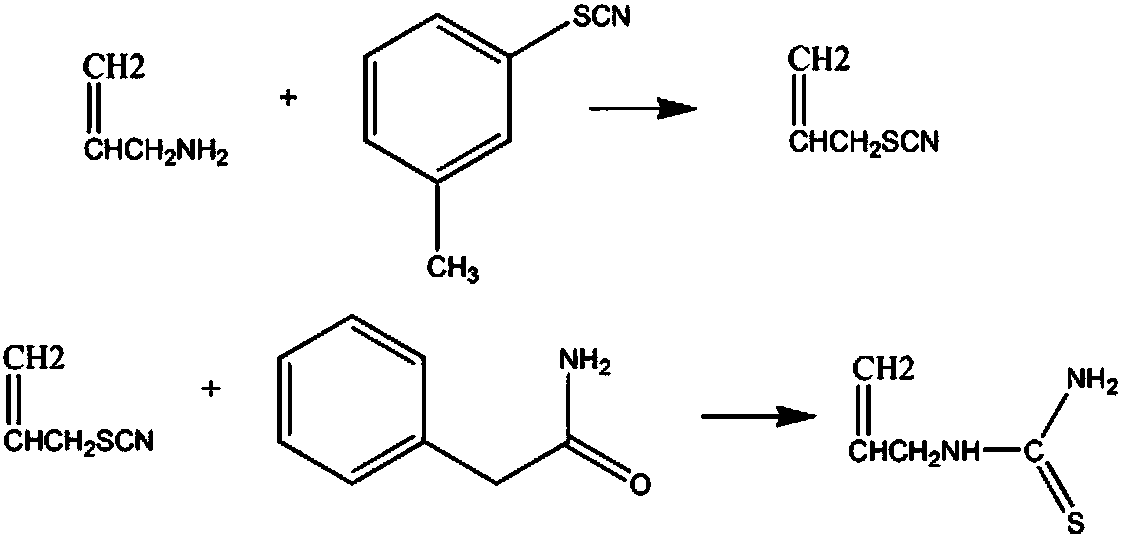
[0011] Add 5L mass fraction of 10% sodium chloride solution, 4mol of 3-methylisothiocyanate benzene in the reaction vessel, increase the solution temperature to 60°C, add 5mol of allylamine and 6L of 15% acetone solution , reflux for 90min, stand to separate layers, take out the oil layer, wash 5 times with potassium bromide solution to obtain propylene isothiocyanate, dehydrate with dehydrating agent, filter, distill the filtrate under reduced pressure at 20kPa, collect the fraction at 80°C to obtain thiocyanate Propyl thiocyanate; add the obtained propylene thiocyanate to 5mol phenylacetamide, add 2L aqueous solution, raise the temperature of the solution to 50°C, react for 50min, lower the temperature of the solution to 10°C, precipitate crystals, filter, and the mass fraction is 70 % toluene solution, 85% butanone solution by mass fraction, and dehydration with anhydrous potassium carbonate dehydrating agent to obtain 408.32 g of finished product propenylthiourea, with a yi...
example 2
[0013] Add 5L mass fraction of 13% sodium chloride solution, 4mol of 3-methylisothiocyanate benzene in the reaction vessel, increase the solution temperature to 62°C, add 5.5mol of allylamine and 6L of 17% acetone Solution, reflux for 110min, stand to separate layers, take out the oil layer, wash 6 times with potassium bromide solution to obtain propylene isothiocyanate, dehydrate with a dehydrating agent, filter, and distill the filtrate under reduced pressure at 25kPa, collect the fraction at 83°C to obtain sulfur Propylene cyanate; add the obtained propenyl thiocyanate to 5.5mol phenylacetamide, add 2L aqueous solution, raise the solution temperature to 55°C, react for 60min, lower the solution temperature to 12°C, precipitate crystals, filter, mass fraction Wash with 75% toluene solution, wash with 87% butanone solution by mass fraction, and dehydrate with anhydrous calcium sulfate dehydrating agent to obtain 426.88 g of finished allylthiourea with a yield of 92%.
example 3
[0015] Add 5L mass fraction of 16% sodium chloride solution, 4mol of 3-methylisothiocyanate benzene in the reaction vessel, increase the solution temperature to 65°C, add 6mol of allylamine and 6L of 20% acetone solution , reflux for 120min, stand for stratification, take out the oil layer, wash 7 times with potassium bromide solution to obtain propylene isothiocyanate, dehydrate with a dehydrating agent, filter, and distill the filtrate under reduced pressure at 30kPa, collect the fraction at 86°C to obtain thiocyanate Propyl thiocyanate; add the obtained propylene thiocyanate to 6mol phenylacetamide, add 2L aqueous solution, raise the temperature of the solution to 60°C, react for 70min, lower the temperature of the solution to 15°C, precipitate crystals, filter, and the mass fraction is 80 % toluene solution, washing with 90% butanone solution by mass fraction, and dehydration with anhydrous potassium carbonate dehydrating agent to obtain 436.16 g of finished allylthiourea, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com