Finasteride lyotropic liquid crystal gel preparation precursor and preparation method thereof
A technology for finasteride and gel preparation, which is applied in the field of finasteride lyotropic liquid crystal gel preparation precursor and its preparation field, can solve the problems of inability to completely remove organic solvent, low bioavailability, enhanced tissue acidity and the like , to achieve the effect of prolonging the action time of the drug, good biocompatibility and easy injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the present invention is to use a high-speed stirrer, add phospholipids, glycerides and finasteride and mix them initially at a speed of 600-800rpm, then add a cosolvent and continue to stir for 20-30min at a speed of 1000-1500rpm, and then ultrasonicate while vacuuming After stirring for 20-30 minutes, the precursor of the finasteride lyotropic liquid crystal gel preparation is obtained. If necessary, the photosensitizer can be added together with the co-solvent, then stirred at high speed and vacuumed and ultrasonically stirred to obtain a finasteride lyotropic liquid crystal gel preparation precursor containing the photosensitizer. Finally, it was filled with nitrogen, sealed, and stored at -4°C.
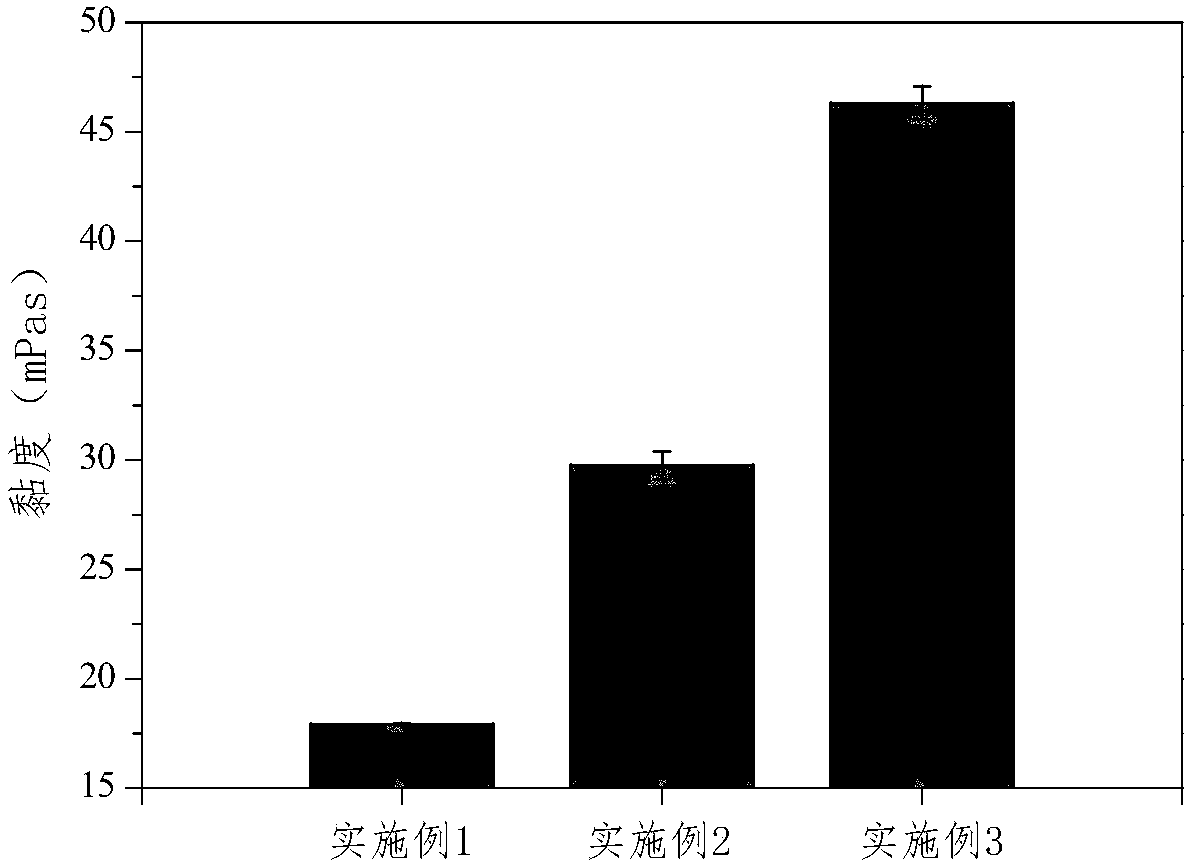
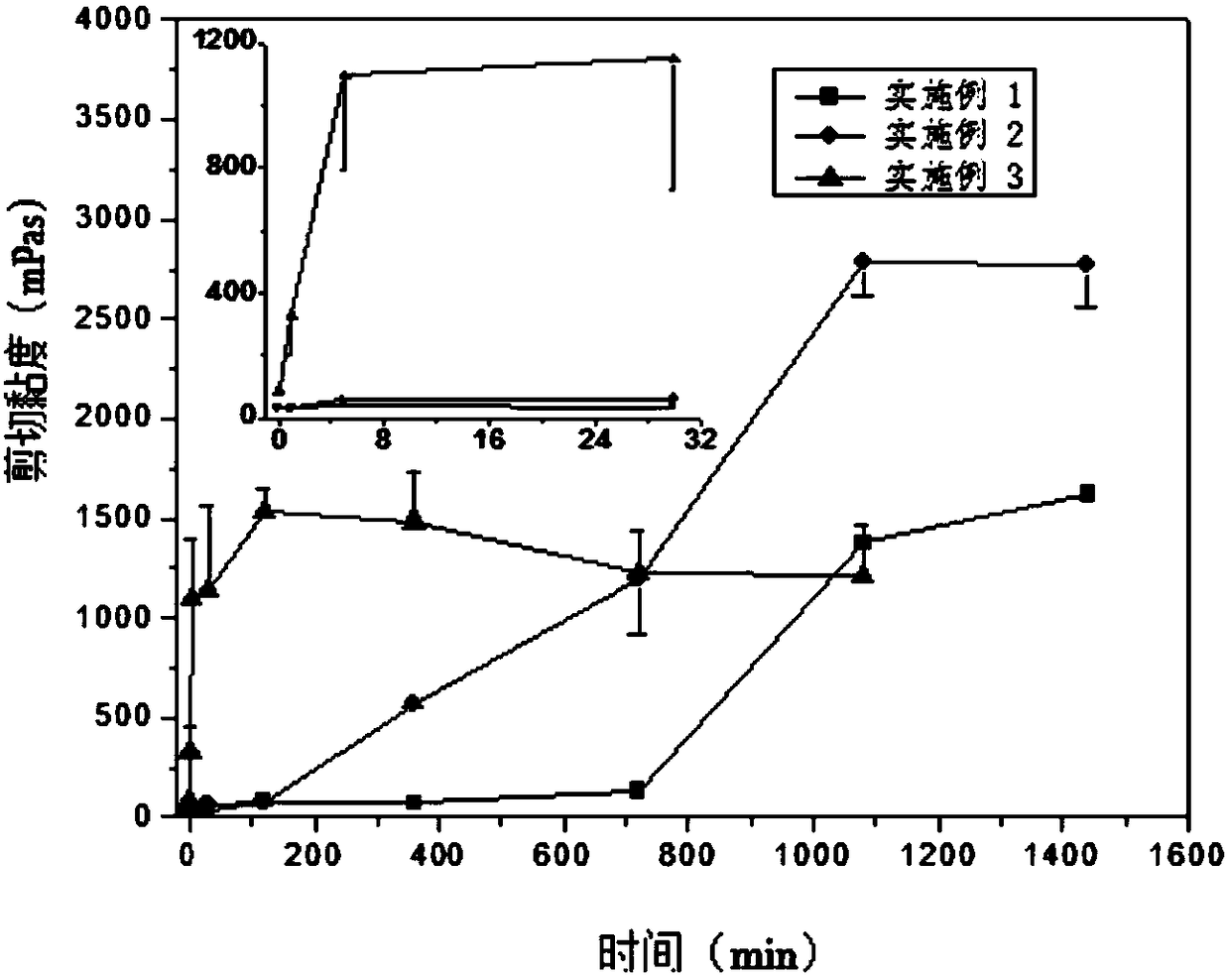
[0044] The viscosity change test method of the finasteride lyotropic liquid crystal gel preparation precursor prepared by the present invention under different shear rates is as follows: use the Malvern Kinexus rotational rheometer to first measur...
Embodiment 1
[0051] This example provides a precursor of finasteride lyotropic liquid crystal gel preparation, which is prepared from the following raw materials in weight percentage: phosphatidylcholine 45%, glyceryl dioleate 49.9%, absolute ethanol 5%, Finasteride 0.1%.
[0052] The appearance of the product obtained in this example is a light yellow transparent solution, which shows that each raw material has good compatibility and is evenly mixed. There is no delamination phenomenon after storage at -4°C for 6 months and centrifugation treatment, indicating that the product has good storage stability.
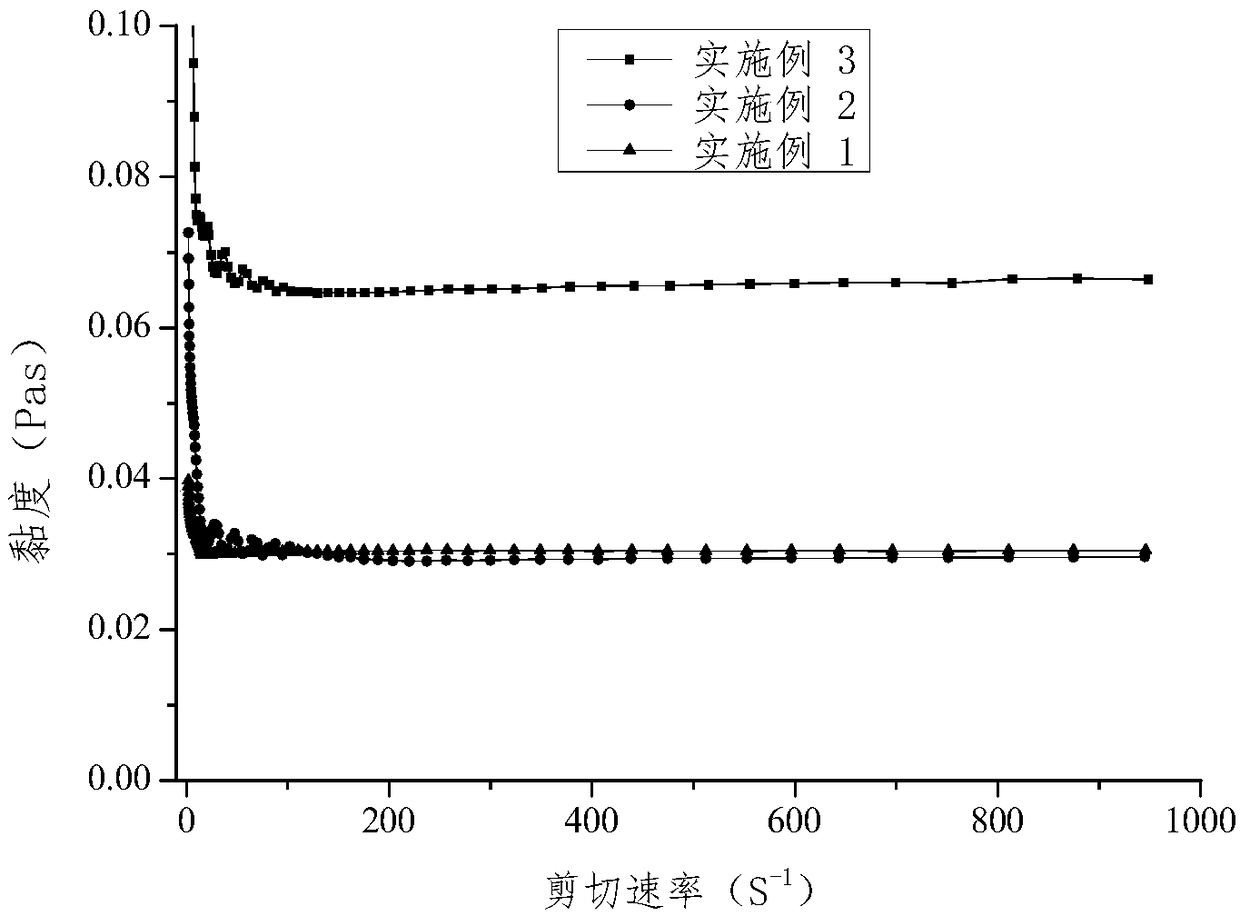
[0053] The viscosity change graph of the finasteride lyotropic liquid crystal gel preparation precursor prepared in Example 1 at different shear rates is shown in figure 1 shown. It was found that when the viscosity of the prepared finasteride lyotropic liquid crystal gel preparation precursor reaches a certain shear rate, the shear viscosity remains unchanged and is not affected by t...
Embodiment 2
[0059] This example provides a precursor of finasteride lyotropic liquid crystal gel preparation, which is prepared from the following raw materials in weight percentage: phosphatidylcholine 80%, glyceryl dioleate 10%, peanut oil 9%, finasteride Andramine 1%.
[0060] The appearance of the product obtained in this example is a light yellow transparent solution, which shows that each raw material has good compatibility and is evenly mixed. There is no delamination phenomenon after storage at -4°C for 6 months and centrifugation treatment, indicating that the product has good storage stability.
[0061] The viscosity change diagram of the finasteride lyotropic liquid crystal gel preparation precursor under different shear rates in this embodiment is shown in figure 1 As shown, the shear stress has nothing to do with the shear speed, and its viscosity is constant, indicating that the precursor of the finasteride lyotropic liquid crystal gel preparation belongs to the plastic flu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com