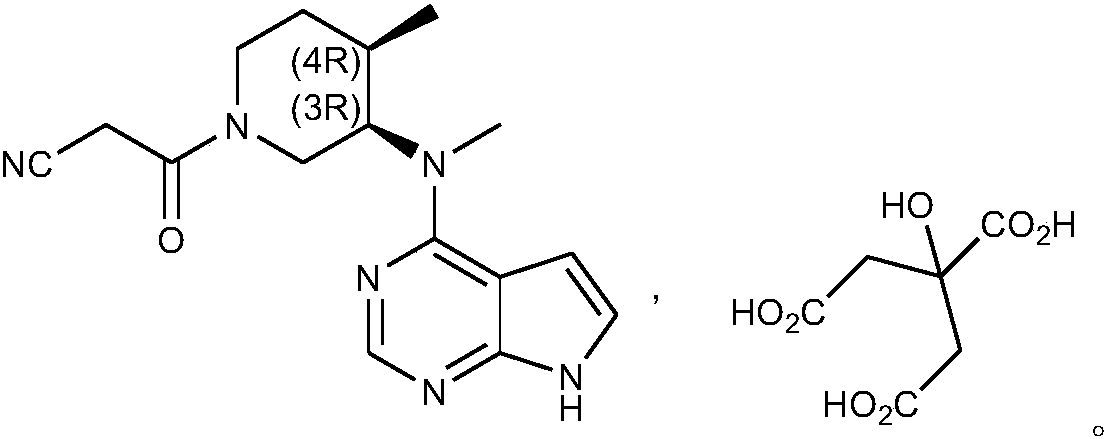
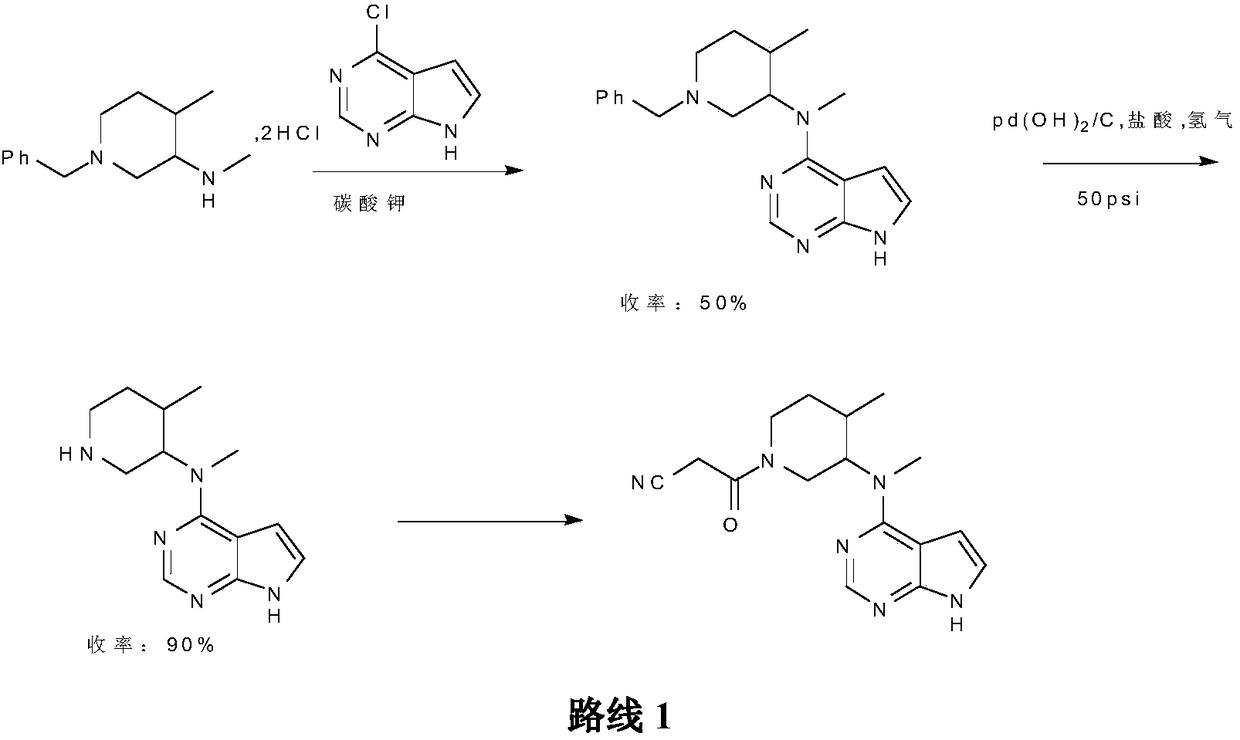
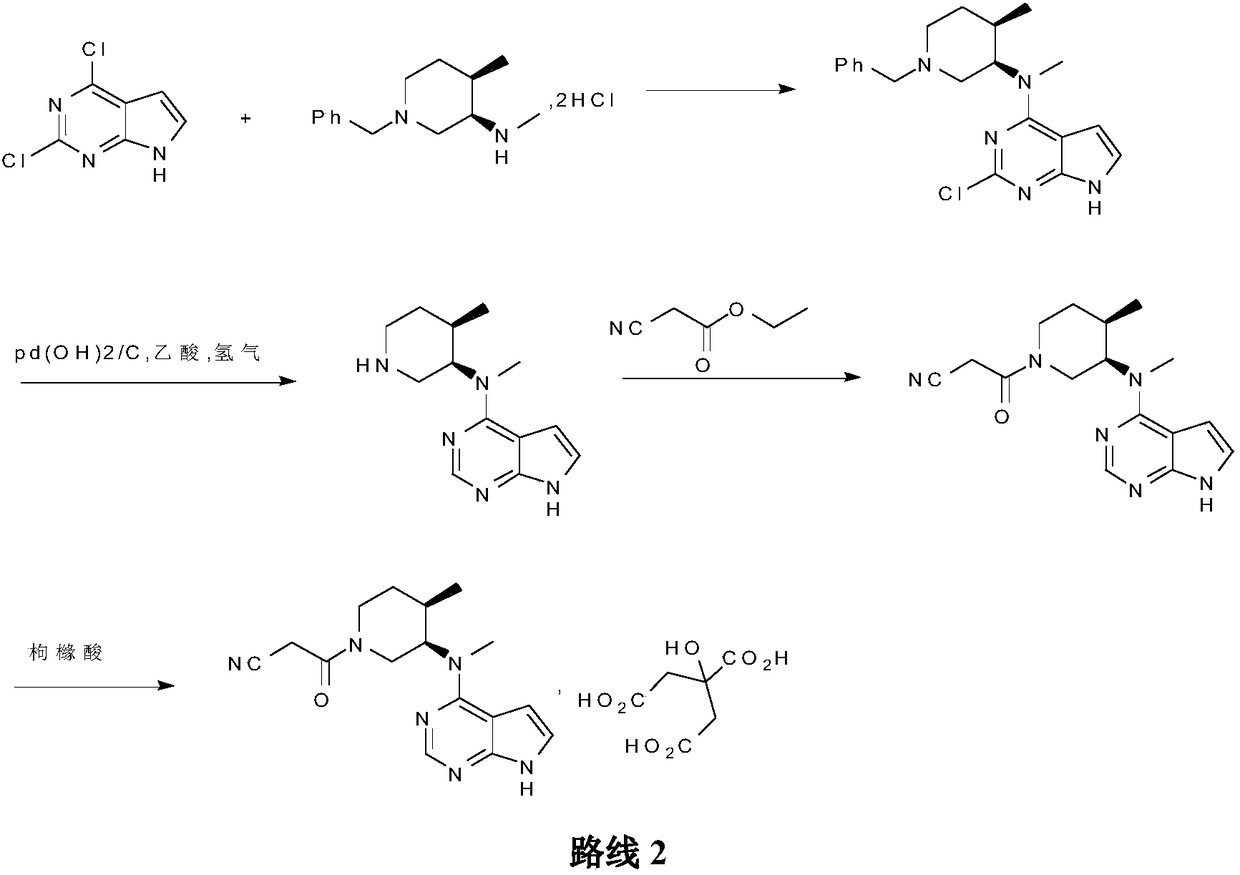
Preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of medicinal chemistry, can solve the problems of flammability and explosion of hydrogen, potential safety hazards, incomplete reaction, etc., and achieve the effects of shortening reaction time, easy operation and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of compound 3
[0039] Add 20g of compound 5 and 13.3g of compound 4 into a 500ml reaction flask, add 266ml of purified water, add a pre-prepared potassium carbonate aqueous solution (20g of potassium carbonate dissolved in 40ml of purified water) under stirring, and heat to reflux. After stirring and refluxing for 10 hours, TLC detection was started until the reaction was complete. Cool down to 20-30°C, add 120ml of dichloromethane, stir and extract, let stand to separate layers, dry, filter, wash to obtain the reaction solution, add n-heptane under stirring at room temperature, cool down to 0-10°C to crystallize for 2-4 hours, Filter and wash. Vacuum drying at 50-60°C for 6 hours gave compound 3, weighing 20.9 g, yield: 82.3%, purity: 99.9%.
Embodiment 2
[0040] Embodiment 2: the preparation of compound 2
[0041]Add 20g of compound 3 into a 500ml beaker, add 300ml of methanol, 7.5g of acetic acid, and 2g of 5% wet palladium carbon and stir until suspended; the reaction module of the microreactor is heated to 30°C, and the cooling module is controlled at 20°C; adjust the flow rate of the feeding pump 30g feed liquid / minute, the mixed feed liquid enters the microreactor from channel A; 15.2g triethylsilane enters the microreactor from channel B, and starts to react after passing through the reaction module, and the reaction liquid is obtained after passing through the cooling module, and the reaction has been detected by TLC (total reaction time 10 minutes), out of the microreactor. Remove the palladium carbon by filtration, at room temperature, add 65g of 10% sodium hydroxide, stir for 1.5 hours, add 80ml of dichloromethane and stir for extraction, stand for layering, add 80ml of dichloromethane to the water phase, stand for la...
Embodiment 3
[0042] Embodiment 3: the preparation of tofacitinib citrate
[0043] Heat up the reaction module of the microreactor to 80°C; add 10g of compound 2 into a 500ml beaker, add 300ml of n-butanol and stir to dissolve, add 9.22g of ethyl cyanoacetate and 6.2g of DBU, and adjust the flow rate of the feeding pump to 30g of feed solution / min Enter the microreactor from channel A; add 15ml purified water and 60ml n-butanol to 21.4g citric acid and stir to dissolve, adjust the flow rate of the feeding pump to 10g feed liquid / min and enter the microreactor from channel B, and react in the reaction module, the total reaction After 10 minutes, the reaction solution was cooled to 20-30°C after leaving the microreactor, stirred and crystallized for 2-3 hours, filtered, washed, and vacuum-dried at 40-50°C for 5 hours to obtain tofacitinib citrate: 19.8g, Yield: 96.4%, purity: 99.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com