Device for detecting lead ions
A polypyrrole and electrode technology, applied in the field of heavy metal detection, can solve the problems of unfavorable heavy metal enrichment, easy stacking, high sensitivity, etc., achieve excellent electrochemical performance, reduce experimental cost, and improve the effect of electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Soak the foamed copper in hydrochloric acid, acetone, distilled water and absolute ethanol in sequence, and ultrasonically clean it to remove oil, oxide layer, insoluble matter, etc. on the foamed copper, and dry the foamed copper in a vacuum oven at 60°C for later use .
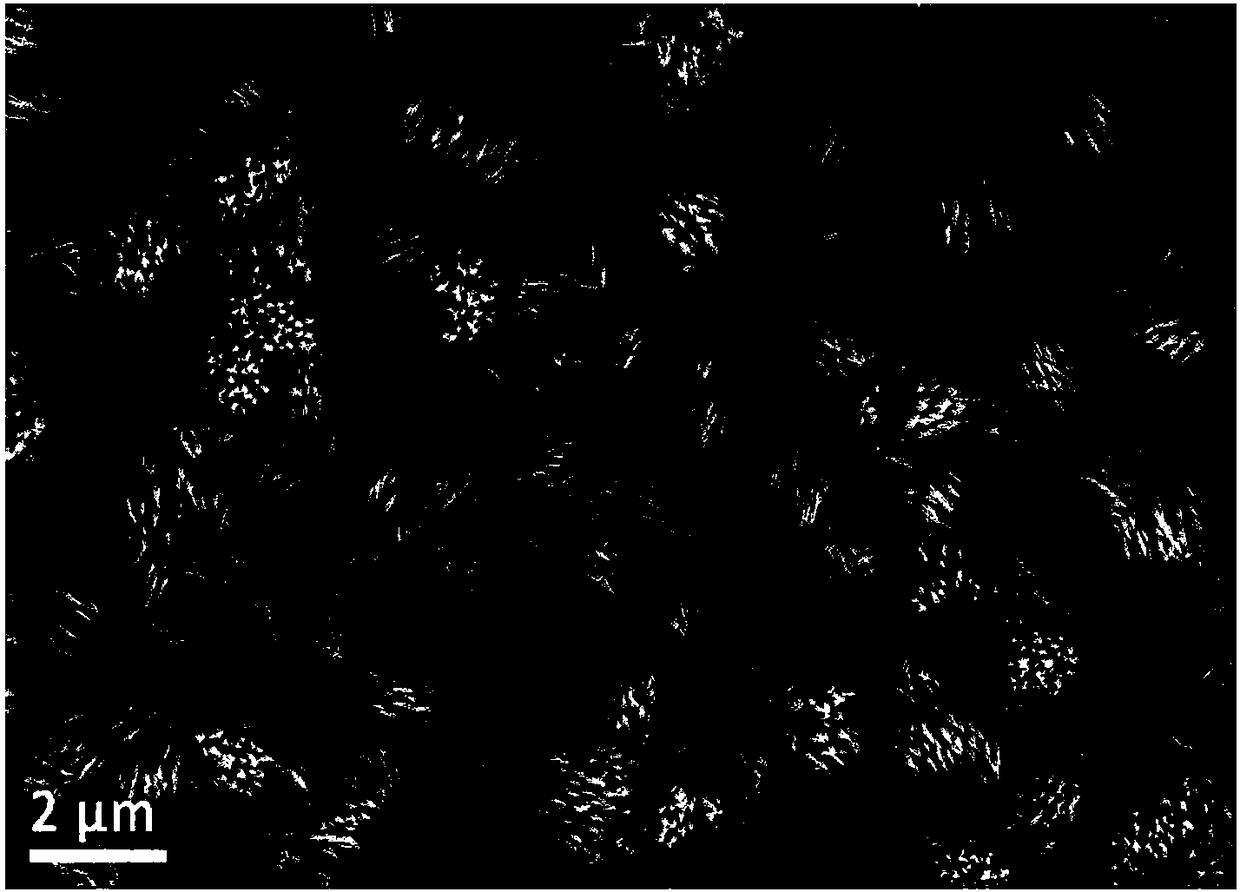
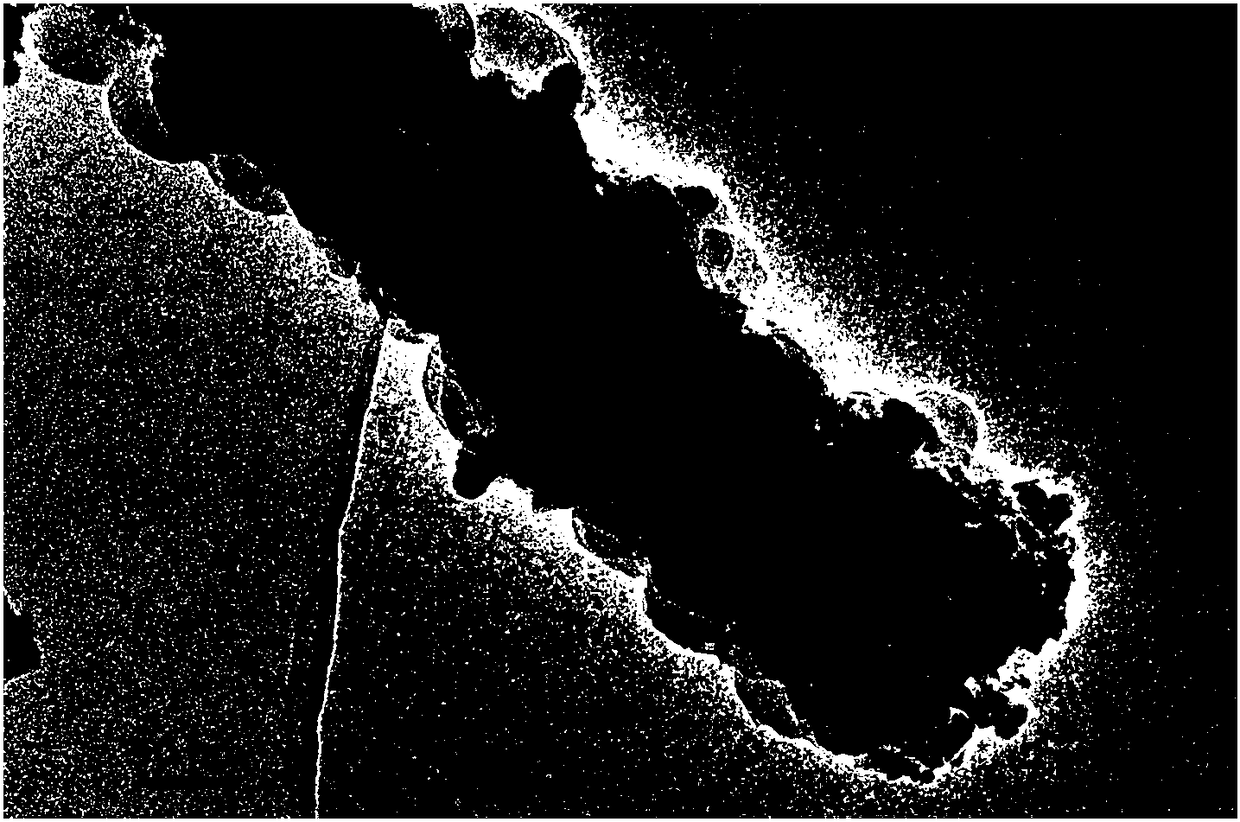
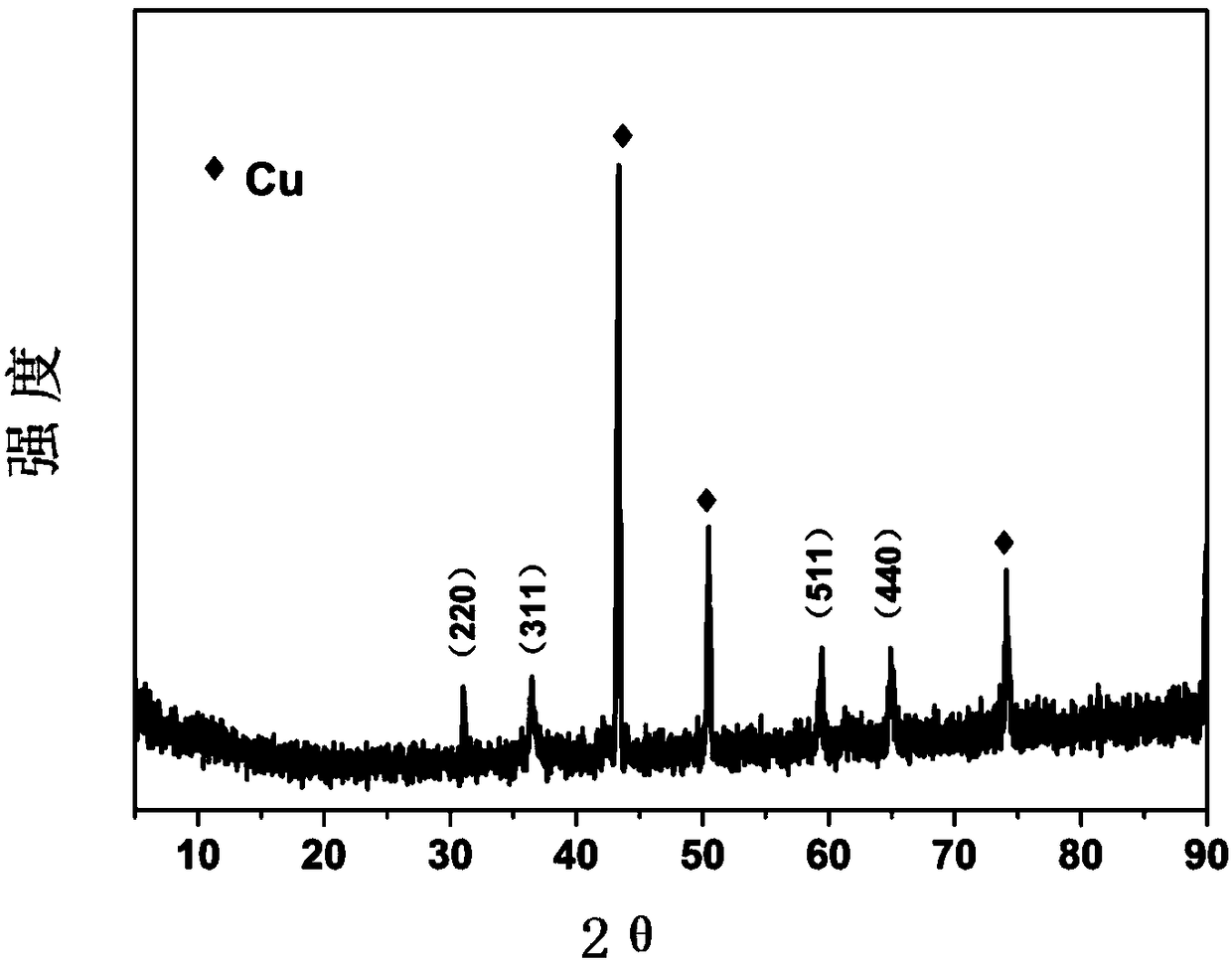
[0034] Cut the pretreated copper foam to a size of 1×2cm 2 As a substrate on which to grow Co 3 o 4 Precursor nanowire array structure. Weigh 0.58g of cobalt nitrate, 0.1389g of ammonium fluoride, and 0.56g of urea, add them into 40ml of distilled water, and stir for 10min to obtain a bright and uniform pink liquid. Pour this pink liquid into a hydrothermal kettle, and suspend the foamed copper in the pink liquid. At 100°C, hydrothermally react for 8 hours. After the reaction, take out the reactor and cool to room temperature. Take out the foamed copper substrate. You can see that there is a pink substance attached to the foamed copper substrate. Rinse repeatedly with distilled water and ethanol, an...
Embodiment 2
[0039] Soak the foamed copper in hydrochloric acid, acetone, distilled water and absolute ethanol in sequence, and ultrasonically clean it to remove oil, oxide layer, insoluble matter, etc. on the foamed copper, and dry the foamed copper in a vacuum oven at 60°C for later use .
[0040] Cut the pretreated copper foam to a size of 1×2cm 2 As a substrate on which to grow Co 3 o 4 Precursor nanowire array structure. Weigh 0.29g of cobalt nitrate, 0.1389g of ammonium fluoride, and 0.3g of urea, add them into 40ml of distilled water, stir for 10min, and obtain a bright and uniform pink liquid. Pour this pink liquid into a hydrothermal kettle, and suspend the foamed copper in the pink liquid. At 120°C, hydrothermally react for 5 hours. After the reaction, take out the reactor and cool to room temperature. Take out the foamed copper substrate. It can be seen that a pink substance is attached to the foamed copper substrate. Rinse repeatedly with distilled water and ethanol, and dr...
Embodiment 3
[0046] Soak the foamed copper in hydrochloric acid, acetone, distilled water and absolute ethanol in sequence, and ultrasonically clean it to remove oil, oxide layer, insoluble matter, etc. on the foamed copper, and dry the foamed copper in a vacuum oven at 70°C for later use .
[0047] Cut the pretreated copper foam to a size of 1×2cm 2 As a substrate on which to grow Co 3 o 4 Precursor nanowire array structure. Weigh 0.87g of cobalt nitrate, 0.074g of ammonium fluoride, and 0.3g of urea, add them into 40ml of distilled water, stir for 20min, and obtain a bright and uniform pink liquid. Pour this pink liquid into a hydrothermal kettle, and suspend the foamed copper in the pink liquid. At 80°C, hydrothermally reacted for 8 hours. After the reaction, the reactor was taken out to cool to room temperature, and the copper foam substrate was taken out. A pink substance was attached to the copper foam substrate. Rinse repeatedly with distilled water and ethanol, and dry at room ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com