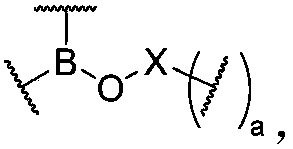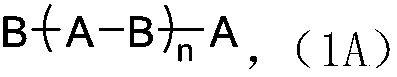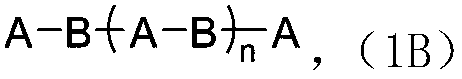Physical phase-split dynamic polymer and application thereof
A polymer and dynamic technology, applied in the field of physical phase-separated dynamic polymers, can solve problems such as lack of dynamics, inability to use elastomers, and poor mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0270] A preferred preparation method of a dynamic polymer ionic liquid gel of the present invention includes but is not limited to the following steps: blending the raw materials for preparing dynamic polymers with ionic liquids, so that the mass fraction of raw materials for preparing dynamic polymers is 0.5- 70%, polymerization, coupling or other types of chemical reactions are carried out through the appropriate means, and after the reaction is completed, a dynamic polymer ionic liquid gel is produced. The preferred preparation method of another dynamic polymer ionic liquid gel of the present invention includes but not limited to the following steps: swelling the dynamic polymer in a solvent containing ionic liquid, so that the mass fraction of the dynamic polymer is 0.5-70% After fully swelling, the solvent is removed to form a dynamic polymer ionic liquid gel. The above-mentioned ionic liquid is generally composed of organic cations and inorganic anions. As an example, t...
Embodiment 1
[0331] Commercially available styrene-butadiene-styrene triblock copolymer (SBS), 3-mercaptopropionic acid, and photoinitiator benzyldimethylketal (BDK) were reacted in tetrahydrofuran to maintain polybutadiene The molar ratio of alkenyl to 3-mercaptopropionic acid and BDK in the chain segment is about 50:5:1, and the modified SBS containing side carboxyl groups in the polybutadiene chain segment is obtained. Gained modified SBS and 2-aminomethylbenzeneboronic acid, 4-aminophenylboronic acid use 2-ethoxyl-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ) as a condensing agent to keep the modified The molar ratio of carboxyl to 2-aminomethylphenylboronic acid and 4-aminophenylboronic acid in SBS is 2:1:1, in the dark at room temperature, in a mixed solvent with a volume ratio of dichloromethane / methanol of 2:1 After reacting for 16 hours, the modified SBS containing pendant aminomethylphenylboronic acid groups and pendant phenylboronic acid groups in the polybutadiene segment was ob...
Embodiment 2
[0336] Allyl boronic acid pinacol ester and equimolar equivalent of mercaptosuccinic acid were blended in tetrahydrofuran, and reacted under ultraviolet light in the presence of photoinitiator BDK to obtain compound 2a.
[0337] Compound 2a was reacted with excess polycaprolactone terminated by hydroxyl groups at both ends, and under the catalysis of dicycloethylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP), a hydroxyl-terminated polycaprolactone at both ends was obtained. A polyester segment containing a pendant organoboronic acid cyclic ester group.
[0338]
[0339] Use benzoyl peroxide (BPO) as the initiator and mercaptoacetic acid as the chain transfer agent to initiate the polymerization of 4-vinylpyridine at 90-100°C, keeping the molar ratio of initiator, monomer and chain transfer agent at 1:30:1 , to obtain single-terminal carboxyl-terminated poly(4-vinylpyridine).
[0340] Mixing 1 molar equivalent of the obtained copolymer segment with 2 molar equivalent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com