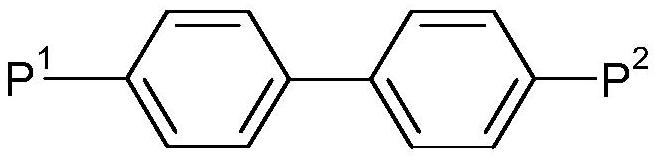
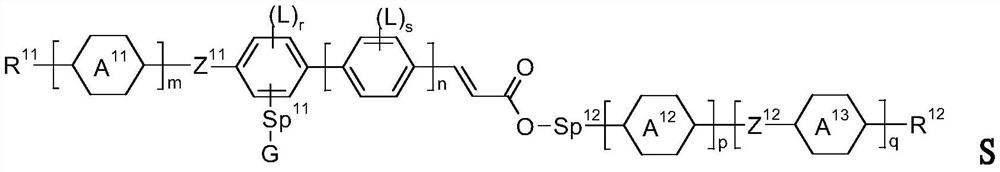
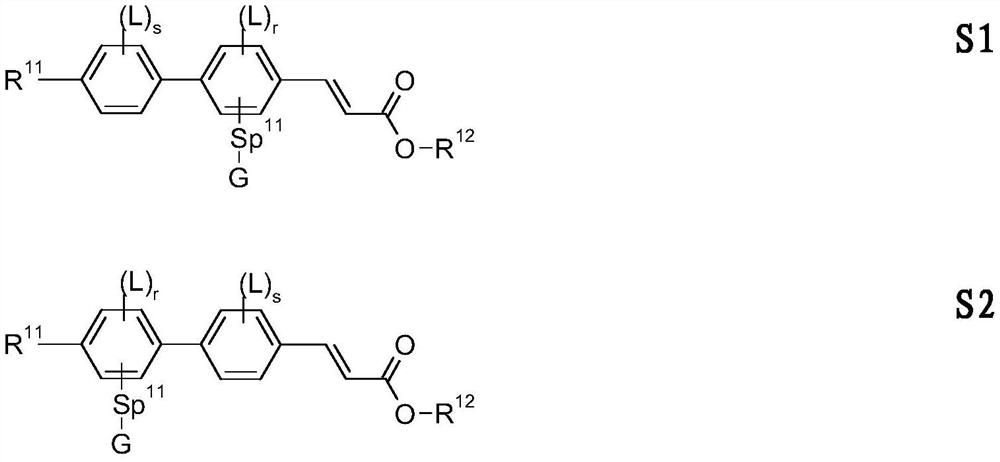
Cinnamic Acid Derivatives
A mixture and compound technology, applied in the field of cinnamic acid derivatives, can solve problems such as inhomogeneity, pollution, electrostatic discharge, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0534] Hereinafter, the present invention will be explained in more detail and specifically with reference to Examples, however, the Examples are not intended to limit the present invention.
[0535] Synthesis Example 1: (E)-3-[3-(2-hydroxyethoxy)-4-(4-pentylbiphenyl)]prop-2-enoic acid ethyl ester
[0536] The starting material 2-fluoro-4-iodo-1-(4-pentyl-phenyl)-benzene was prepared according to literature procedures by combining 5-pentylphenylboronic acid with 4-bromo-2-fluoro-1 -Iodine-benzene Suzuki coupling followed by bromine-lithium exchange and reaction of the lithiated intermediate with iodine.
[0537] Stage 1: 2-[5-iodo-2-(4-pentylphenyl)phenoxy]ethanol
[0538]
[0539] Triethylene glycol dimethyl ether (100 ml), dibenzo-18-crown-6 (1.3 g, 3.6 mmol) and sodium hydride (8 g, 60% dispersion in oil, 200 mmol) were stirred at room temperature. Anhydrous ethylene glycol (40ml, 720mmol) was added dropwise over 30 minutes, keeping the temperature below 60°C. 2-Fluor...
Embodiment 2
[0543] Example 2: (E)-3-[4-(4-pentylphenyl)-3-(3-trimethoxysilylpropoxy)-phenyl]prop-2-enoic acid ethyl ester
[0544] Step 1: (E)-3-[3-allyloxy-4-(4-pentylphenyl)phenyl]prop-2-enoic acid ethyl ester
[0545]
[0546] 2-Fluoro-4-iodo-1-(4-pentyl-phenyl)-benzene was reacted with sodium hydroxide in triethylene glycol under the same reaction conditions as described in Example 1 to give 5- Iodo-2-(4-pentylphenyl)phenol.
[0547] 5-iodo-2-(4-pentylphenyl)phenol (0.5g, 1.48mmol), allyl bromide (0.14ml, 1.62mmol), potassium carbonate (0.3g, 2.2mmol) and Butanone (3ml) was heated for 3 hours. The mixture was cooled and the solvent was removed in vacuo. The residue was dissolved in dichloromethane and purified by vacuum flash chromatography on silica (40 g) eluting with dichloromethane. Fractions containing product were combined and the solvent was removed in vacuo to give ethyl (E)-3-[3-allyloxy-4-(4-pentylphenyl)phenyl]prop-2-enoate .
[0548] Step 2: (E)-3-[4-(4-Pentylphen...
Embodiment 3
[0553]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com