Purification and ion control method of teriparatide acetate
A technology of teriparatide and control method, which is applied in the field of purification and ion control of teriparatide acetate, can solve the problem that the content of acetate group is not described, the content of impurities and ions is not clear, the content of single impurities is not specified, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Sample treatment: Dissolve each gram of crude teriparatide in 50ml of acetonitrile aqueous solution with volume ratio: acetonitrile: water = 5:95, sonicate until completely dissolved, filter through a 0.45 μm filter membrane, and collect the filtrate for later use.
[0041] 2. The first purification:
[0042] Purification conditions: Chromatographic column: DAC-20 dynamic axial pressurized column with octadecylsilane bonded silica gel as the stationary phase, column diameter and packing length: 20*25cm. Mobile phase A: ammonium bicarbonate aqueous solution with a molar concentration of 0.1 mol / L, adjust the pH to 8.0 with ammonia water; phase B: acetonitrile. Flow rate: 1200ml / min. Check wavelength 220nm. Gradient: B%: 10-30%, 45min, the injection volume is 80g.
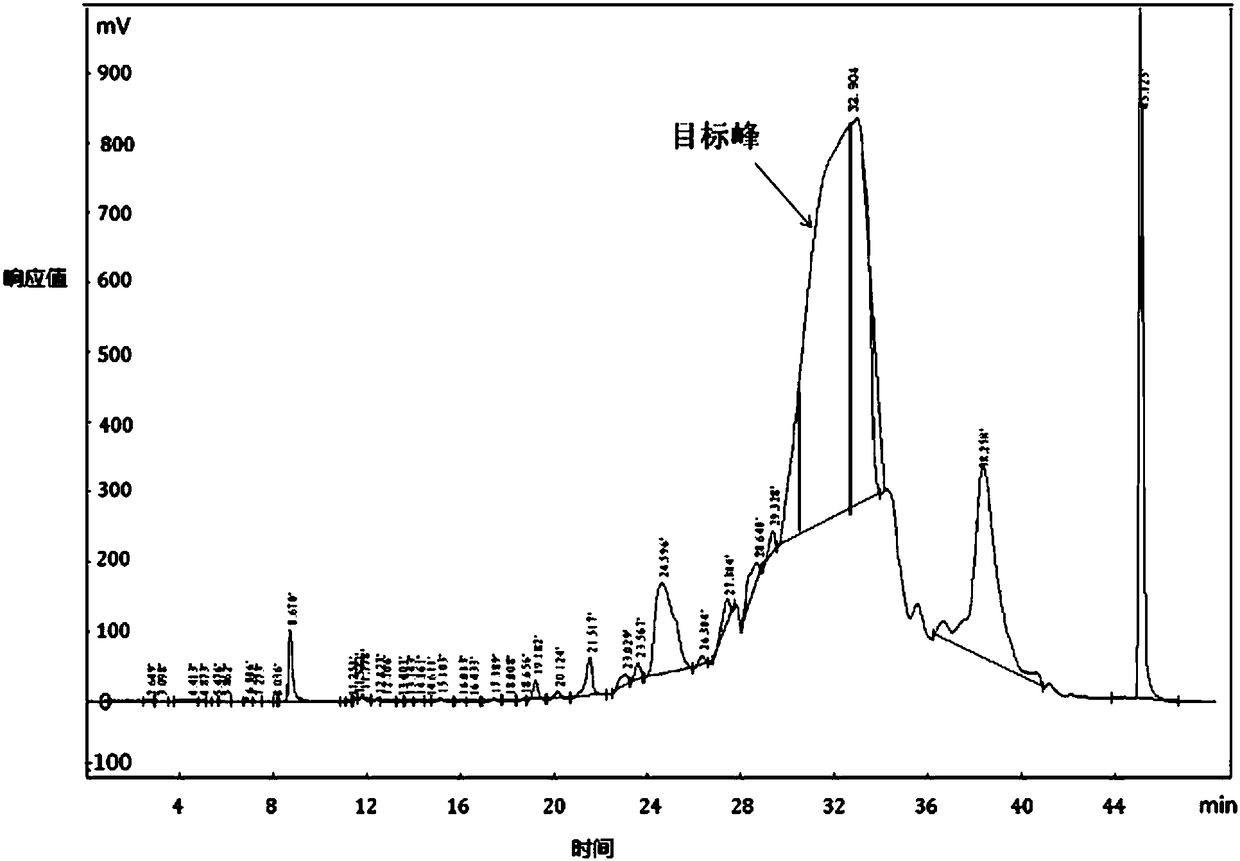
[0043] Purification process: equilibrate the chromatographic column with mobile phase A and then load the sample, with a sample volume of 4L sample solution. The linear gradient was eluted for 45min, and ...
Embodiment 2
[0053] 1. Sample treatment: Dissolve each gram of crude teriparatide in 50ml of acetonitrile aqueous solution with volume ratio: acetonitrile: water = 5:95, sonicate until completely dissolved, filter through a 0.45 μm filter membrane, and collect the filtrate for later use.
[0054] 2. The first purification:
[0055] Purification conditions: Chromatographic column: DAC-20 dynamic axial pressurized column with octadecylsilane bonded silica gel as the stationary phase, column diameter and packing length: 20*25cm. Mobile phase A: ammonium bicarbonate aqueous solution with a molar concentration of 0.01mol / L, adjusted to pH 7.0 with acetic acid; phase B: acetonitrile. Flow rate: 1200ml / min. Check wavelength 220nm. Gradient: B%: 10% ~ 30%, 45min, the injection volume is 80g.
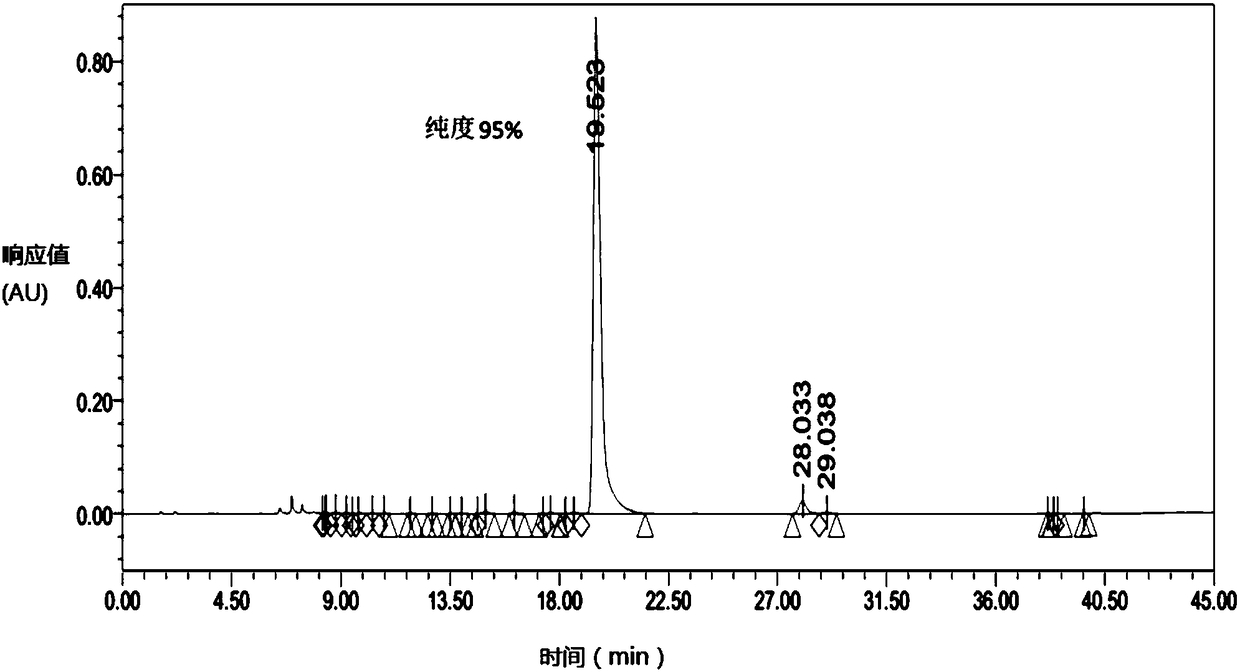
[0056] Purification process: equilibrate the chromatographic column with mobile phase A and then load the sample, with a sample volume of 4L sample solution. The linear gradient was eluted for 45min, and...
Embodiment 3
[0066] 1. Sample treatment: Dissolve each gram of crude teriparatide in 50ml of acetonitrile aqueous solution with volume ratio: acetonitrile: water = 5:95, sonicate until completely dissolved, filter through a 0.45 μm filter membrane, and collect the filtrate for later use.
[0067] 2. The first purification:
[0068] Purification conditions: Chromatographic column: DAC-20 dynamic axial pressurized column with octaalkylsilane bonded silica gel as stationary phase, column diameter and packing length: 20*25cm. Mobile phase A: ammonium bicarbonate aqueous solution with a molar concentration of 0.01 mol / L, adjust the pH to 7.5 with ammonia water; phase B: acetonitrile. Flow rate: 1200ml / min. Check wavelength 220nm. Gradient: B%: 10% ~ 30%, 45min, the injection volume is 80g.
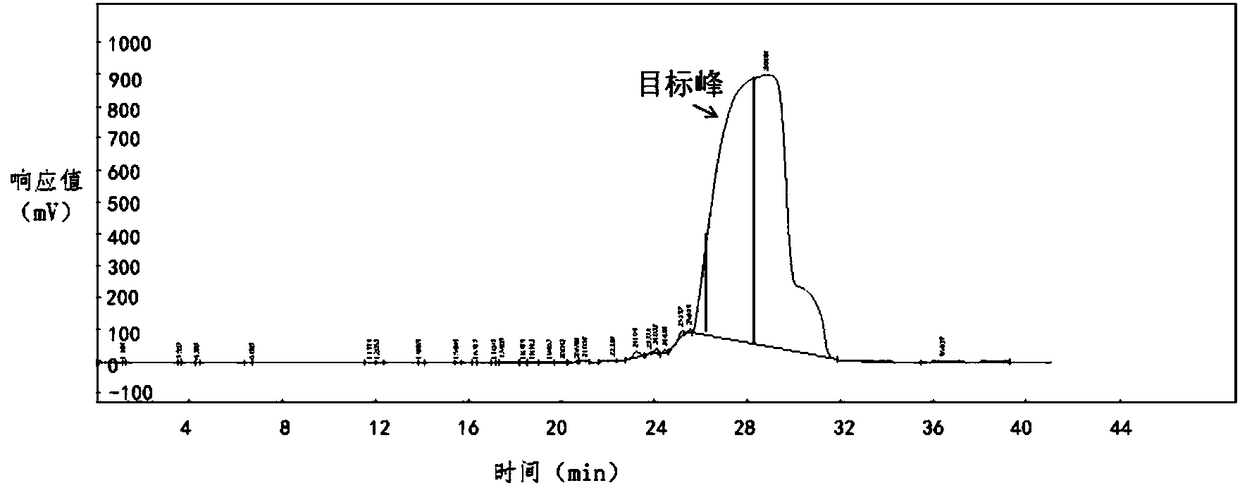
[0069] Purification process: equilibrate the chromatographic column with mobile phase A and then load the sample, with a sample volume of 4L sample solution. The linear gradient was eluted for 45min, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com