Synthesis method and application of triblock multifunctional fused protein based on mussel attachment protein/zwitter-ion polypeptide
A mussel adhesion protein and fusion protein technology, applied in the biological field, can solve problems such as weakening the signal-to-noise ratio of equipment, reducing detection accuracy, and infectious complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
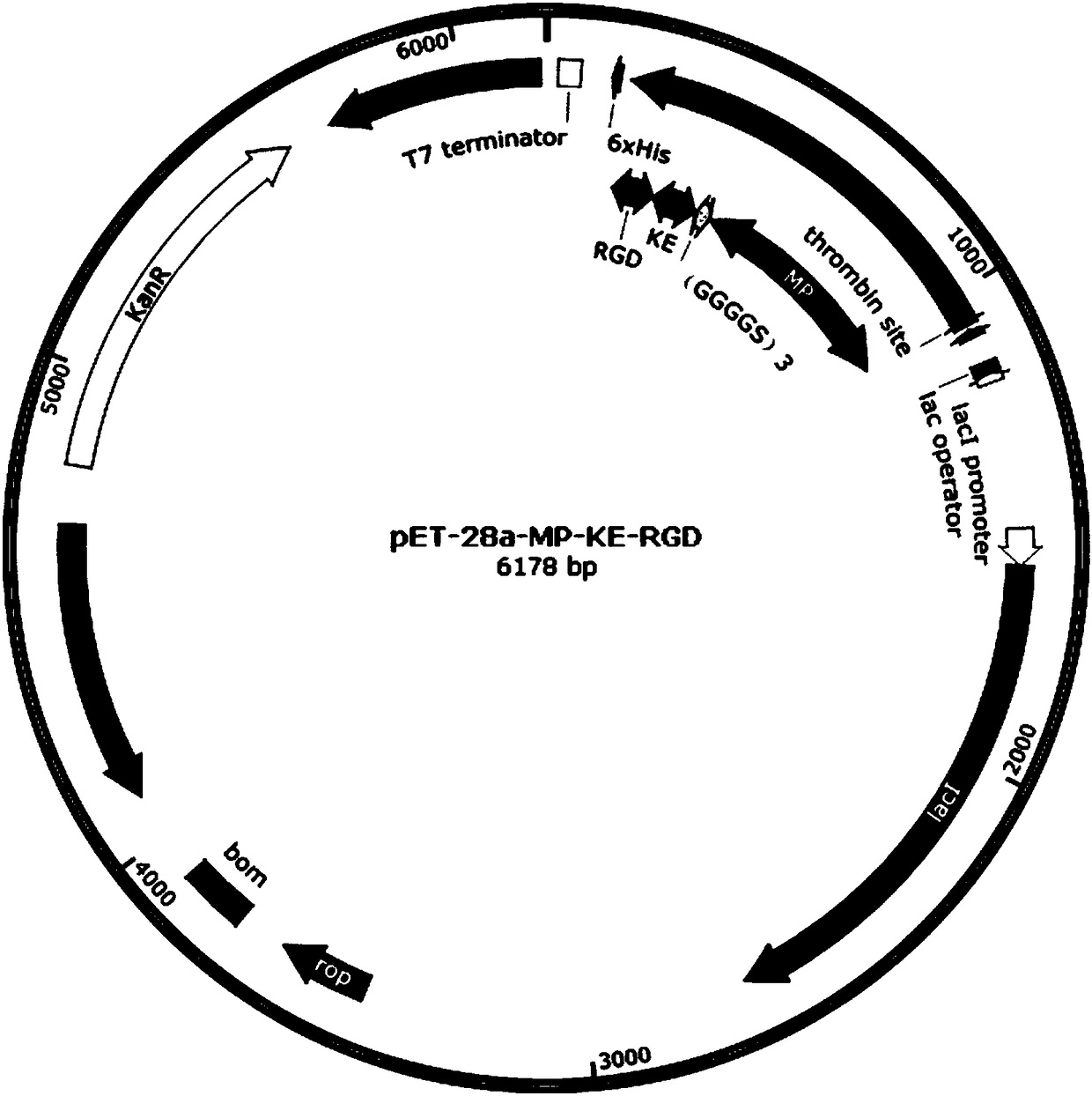
[0059] Embodiment 1: Construction of the recombinant expression vector of MP-KE-RGD triblock multifunctional fusion protein and the construction of bacterial strain
[0060] The preferred amino acid sequence of the MP-KE-RGD triblock multifunctional fusion protein is SEQ ID NO.14, and the preferred nucleotide sequence is shown in SEQ ID NO.18.
[0061] A NdeI restriction enzyme cutting site was added at the 5' end of the SEQ ID NO.18 gene sequence, and a HindIII restriction enzyme cutting site was added at the 3' end. The gene sequence was artificially synthesized and then amplified by PCR. The total reaction volume was 50 μl, in which 2.5 μl of each primer was added at a concentration of 10 μmol / L, and 1 μl of dNTP at a concentration of 10 mmol / L was added. The DNA polymerase used was Phusion High-Fidelity DNApolymerase, 2 U / μl , add 0.5 μl. The reaction conditions were 98°C for 5s, 55°C for 45s, and 72°C for 30s. After 25 cycles, the product was analyzed by 1.0% agarose gel...
Embodiment 2
[0065] Example 2: High expression of MP-KE-RGD triblock multifunctional fusion protein in Escherichia coli
[0066] The successfully transformed Escherichia coli was streaked on a double-resistant LB solid medium plate containing kanamycin and chloramphenicol, and cultured overnight at 37°C for activation. Pick a single colony in a 5 ml test tube containing 50 μg / ml (final concentration) of kanamycin and chloramphenicol sterilized LB liquid medium, and cultivate overnight at 37° C. with shaking at 200 rpm. Transfer Escherichia coli seed solution to a 500ml shake flask at a ratio of 1:100, which contains 200ml sterile LB liquid medium with a final concentration of 50μg / ml kanamycin and chloramphenicol. Measure the growth curve of Escherichia coli with a UV spectrophotometer, and when the concentration of the bacteria solution is OD600=0.8 2h30min after Escherichia coli transfer (IPTG) induces Escherichia coli to express the fusion protein MP-KE-RGD, and continues culturing for...
Embodiment 3
[0067] Example 3: Separation and purification of MP-KE-RGD triblock multifunctional fusion protein in Escherichia coli
[0068] The Escherichia coli fermentation broth was centrifuged at 4°C and 6000rpm for 10 minutes to discard the culture medium, the Escherichia coli cells were washed with PBS buffer three times, and then centrifuged to collect the E. coli cells under the same conditions. Dilute Escherichia coli cells with PBS solution to 40ml / g cell wet weight, and ultrasonically disrupt the bacterial solution in an ice-water bath for 40min with an output power of 200W, crush for 2s, and rest for 3s. The clarified bacterial liquid obtained by crushing was centrifuged at 4°C and 12,000 rpm for 30 minutes to obtain the supernatant and inclusion bodies respectively, and the E. Acrylamide gel (SDS-PAGE) electrophoresis. The fusion protein MP-KE-RGD exists in the supernatant and inclusion bodies at the same time, so the follow-up experiment uses affinity chromatography to separ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com