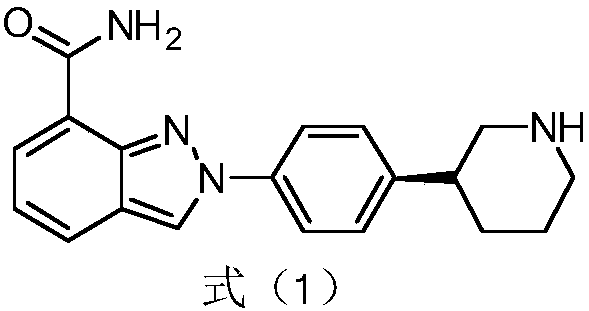
Preparation method of (S)-3-(4-bromophenyl)piperidine as Niraparib intermediate
An intermediate, bromophenyl technology, is applied in the field of preparation of the PARP inhibitor Niraparib intermediate-3-piperidine, which can solve the problems of high production cost and expensive reagents, achieve easy control of operating conditions, reduce production costs, and realize The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
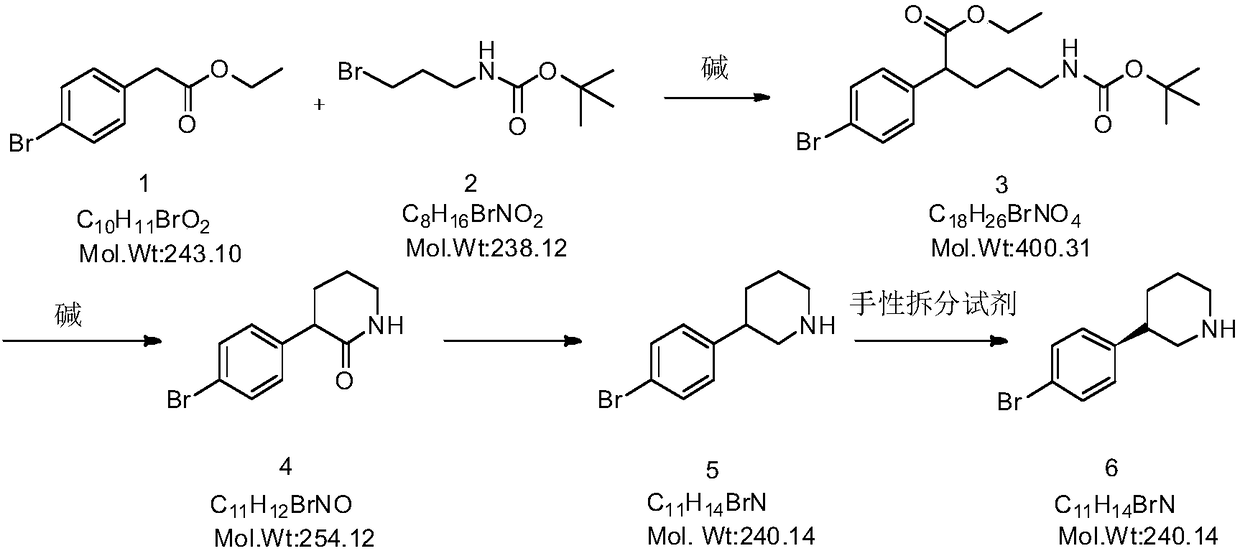
[0030] Embodiment 1 prepares compound 3
[0031]
[0032] Weigh compound 1 (1.20g, 4.9mmol) and dissolve it in 3ml of anhydrous dimethyl sulfoxide, add 211mg of sodium hydride (60%, 5.275mmol) and stir at room temperature for 3min, then weigh N-Boc-3-aminopropyl Bromine (1.05g, 4.411mmol, compound 2) was dissolved in 3ml of anhydrous dimethyl sulfoxide, and slowly added dropwise to the reaction solution. After the dropwise addition, the temperature was raised to 50°C and stirred for 35min. Add saturated ammonium chloride solution to quench the reaction, extract the aqueous phase with ethyl acetate (15ml*3, that is, 15ml each time, three times in total), combine the organic phases, wash with saturated brine (15ml*2), anhydrous sodium sulfate Drying, filtration, concentration under reduced pressure, and column chromatography (eluent: petroleum ether: ethyl acetate = 40:1) gave a white oily substance, the target compound 3 (1.69 g, yield 86%).
[0033] The target product comp...
Embodiment 2
[0037] Embodiment 2 prepares compound 4
[0038]
[0039]Weigh compound 3 (1.69g, 4.22mmol) and dissolve it in 9ml ethyl acetate, add 2ml HCl (it can also be replaced by trifluorobenzenesulfonic acid) and stir at room temperature for 30 minutes, the effect is to remove the amino protective structure, and dissolve it in 25ml after concentrating under reduced pressure. After adding potassium carbonate (0.7g, 5.064mol) to ethanol, reflux at 80°C for 20h. After the reaction, add dilute hydrochloric acid (also can be replaced by sulfuric acid) to neutralize excess alkali, then spin dry the solvent, wash with water, filter and dry to obtain a white solid which is the target compound 4 (0.75 g, yield 70%).
[0040] The target product compound 4 1 The HNMR data are as follows:
[0041] 1 H NMR (500MHz, CDCl 3 )δ:
[0042] 7.57–7.50(m,2H),7.27–7.20(m,2H),6.53(s,1H),3.65(t,J=6.7Hz,1H),3.43(dt,J=12.3,6.8Hz,1H) , 3.26 (dt, J=12.4, 6.8Hz, 1H), 2.28–2.15 (m, 1H), 1.93–1.83 (m, 1H),...
Embodiment 3
[0044] Embodiment 3 prepares compound 5
[0045]
[0046] Weigh compound 4 (0.75g, 2.96mmol) and dissolve it in 15ml THF (tetrahydrofuran), cool down to 0°C, add borane tetrahydrofuran complex (6.65ml, 1.0M in THF, 6.65mmol, CAS: 14044-65-6) Stir overnight at room temperature, then add a few drops of dilute hydrochloric acid to quench the reaction and reflux for 1.5h, evaporate the solvent under reduced pressure and spin dry, add an appropriate amount of sodium hydroxide solution, extract with dichloromethane (25ml*3), combine the organic phases, and saturated chlorination Wash with sodium (25ml*2), dry, filter, concentrate, add 25ml of water and 25ml of hydrochloric acid, reflux at 110°C for 3 hours, add an appropriate amount of sodium hydroxide to adjust the pH to 7, and extract with dichloromethane (30ml*3) , the organic phases were combined, washed with saturated sodium chloride (30ml*2) and concentrated under reduced pressure to obtain a pale yellow solid, the target c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com