Preparation method for high-purity tocopherol succinate salt
A technology of tocopheryl succinate and tocopherol, which is applied in the direction of organic chemistry, can solve the problems of difficult purification of impurities and low purity, and achieve the effects of reducing content, improving purity, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of isophytol:
[0052] Dissolve 15g of farnesyl acetone in 90mL of isopropanol, add 3g of palladium carbon, hydrogen pressure 70kg / cm 3 , 100°C for 3 hours to obtain an intermediate (hexahydrofarnesylacetone).
[0053] In a 500mL round-bottomed three-neck flask with a thermometer inserted, add 230mL (0.23mol, 1.0mol / L) of newly prepared vinyl chloride magnesium Grignard reagent under nitrogen protection, and cool the reaction solution to about 0°C in an ice bath. Stir, slowly add hexahydrofarnesylacetone (40.2g, 0.15mol) dropwise, control the temperature of the reaction solution below 5°C, complete the dropwise addition in 2 hours, continue the reaction for 2 hours, and check that the reaction is complete by gas phase detection, keep adding 40mL in an ice bath Concentrated hydrochloric acid quenched the reaction, adjusted the pH value of the reaction solution to 6-7 with saturated aqueous ammonium chloride solution, precipitated a large amount of solid, fil...
Embodiment 2
[0055] Preparation of dl-alpha-tocopherol:
[0056] In a 2L reaction flask, add 325 mL of butyl acetate, 1 mol of trimethylhydroquinone, 0.2 mol of anhydrous zinc chloride and raise the temperature to 110°C to dissolve completely, then add 0.97 mol of isophytol in batches (prepared in Example 1) , divide water at 110°C, and raise the temperature to 129°C to complete the reaction. Drop to 30°C and add 200mL of water, extract and remove zinc chloride, add 200mL of 0.1% hydrochloric acid to the butyl acetate phase for extraction and washing, concentrate and remove the solvent to obtain dl-α-tocopherol, GC99.4%; refractive index n20 / D: 1.506 ; Specific optical rotation (20°C) -0.03°.
Embodiment 3
[0058] Preparation of tocopheryl succinate and calcium salt thereof:
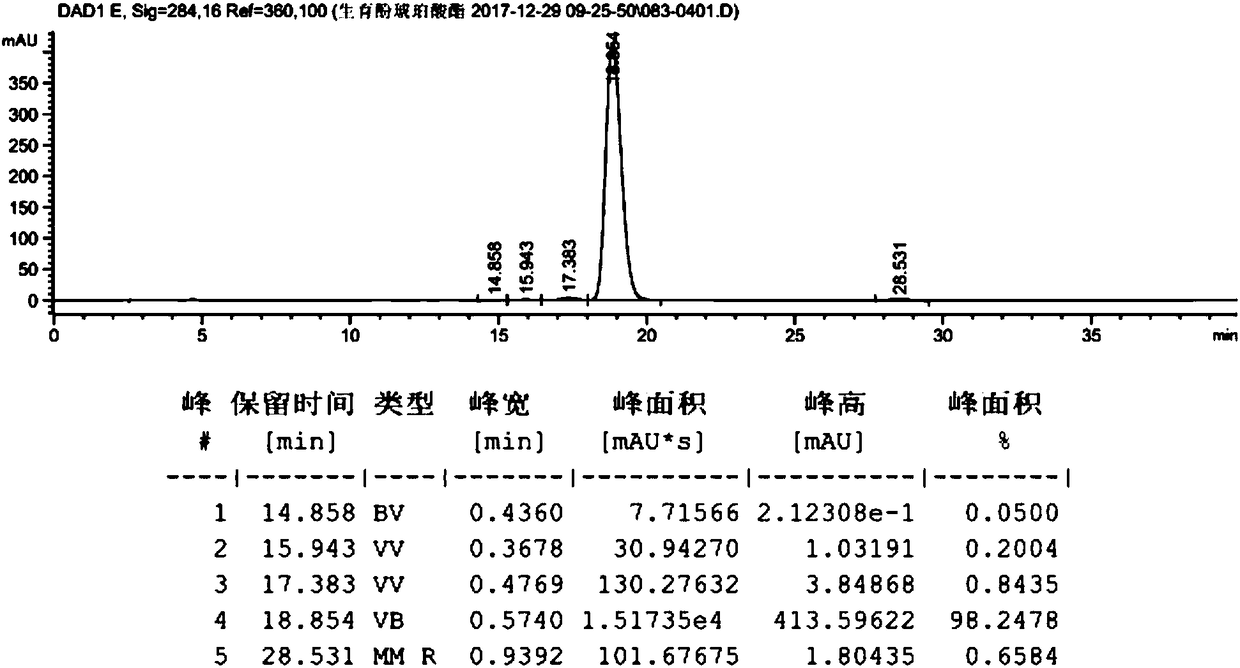
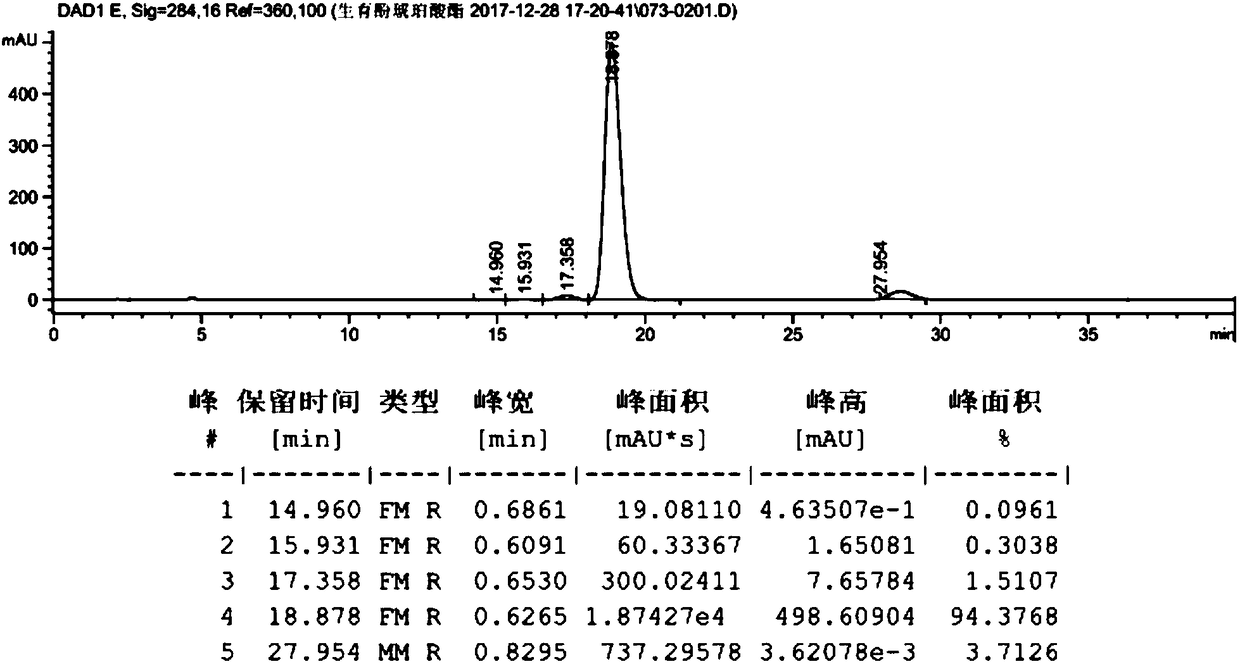
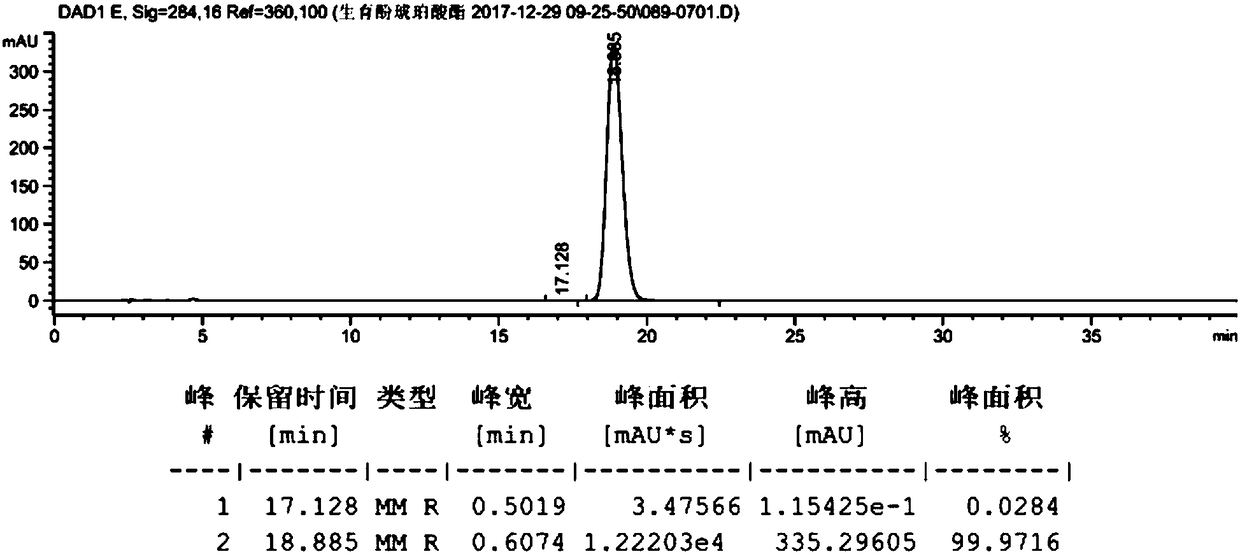
[0059] Take dl-α-tocopherol (10g, 23.22mmol, prepared in Example 2), dissolve it in 100ml of anhydrous methanol, add palladium carbon (0.2g, palladium content 5wt%), and react under 0.3MPa hydrogen pressure for 10h. Filter with celite, wash with 10ml of methanol, and concentrate to obtain 10g of the product. The hydrogenated tocopherol (10g, 23.22mmol) was dissolved in toluene (50mL), and succinic anhydride (4.2g, 41.80mmol) and triethylamine (0.82g, 8.13mmol) were added to react at 60°C for 6h. At room temperature, filter, add 100mL hydrochloric acid (1M), 25mL hydrochloric acid (1M) successively to extract, 25mL water to extract and wash, the organic phase is dried (Na2SO4) and concentrated to give crude product 12.2g, purity 98.2478%, product map as follows figure 1 shown. Dissolve calcium acetate (15.56 g, 0.098 mol) in 106 ml of water, and then add 185 ml of anhydrous methanol to the system to prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com