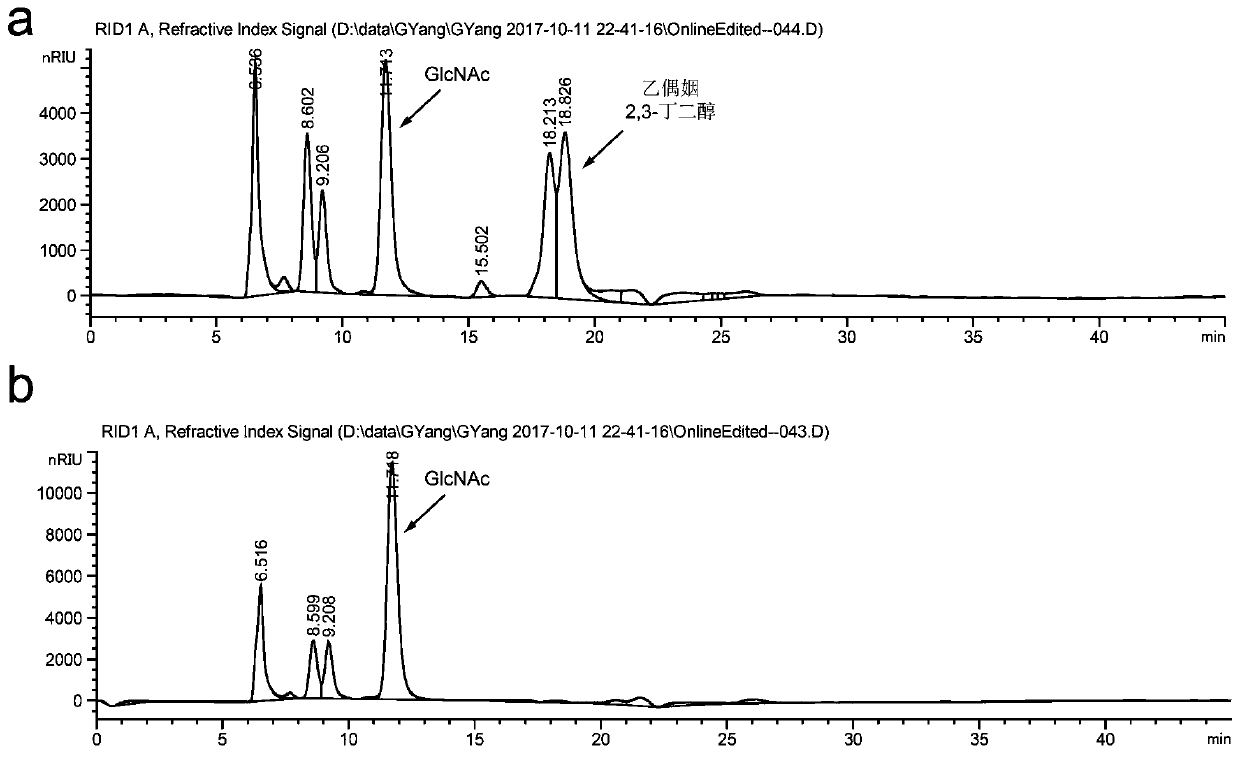
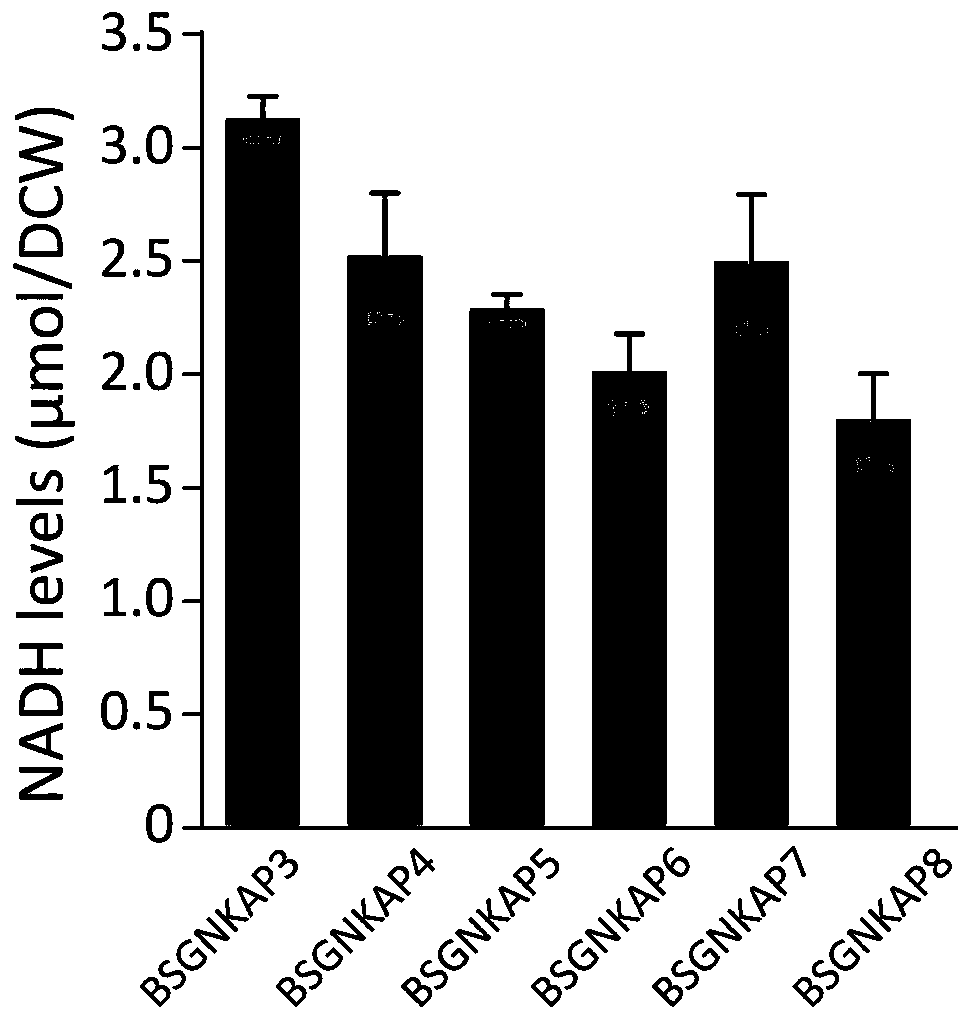
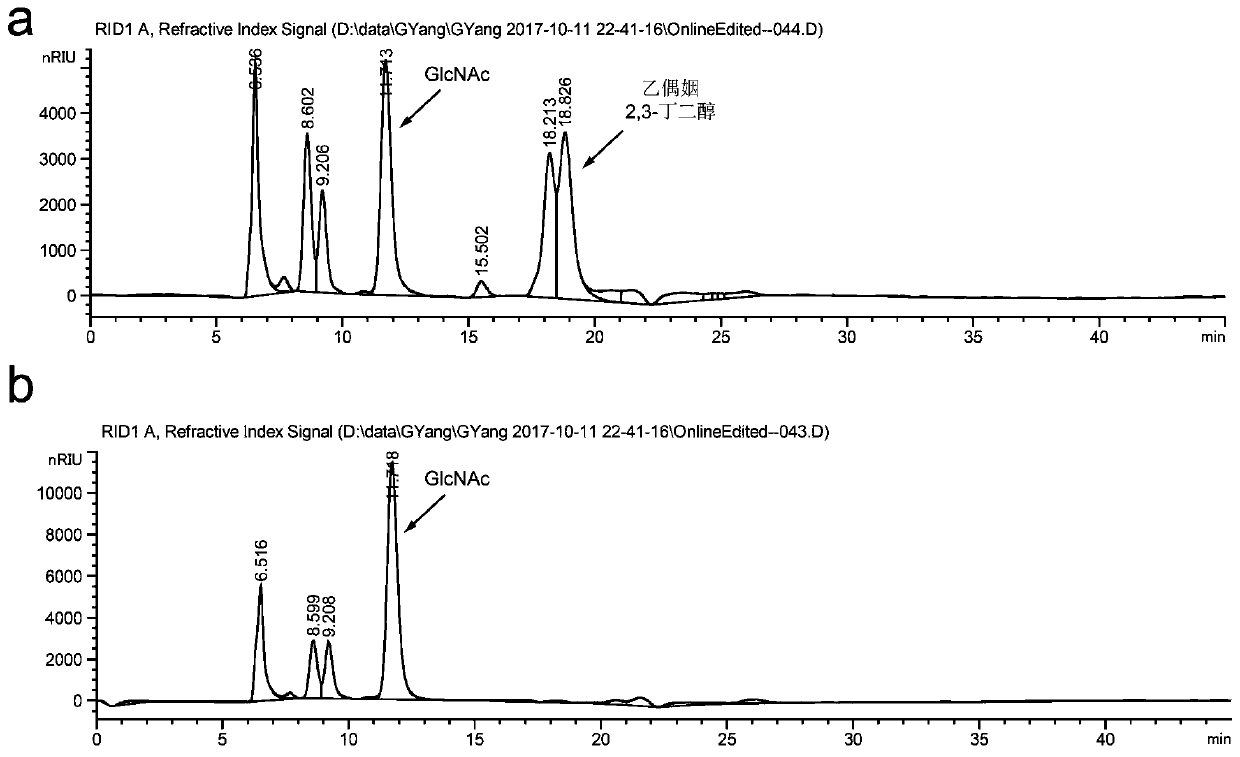
A method for promoting the synthesis of acetylglucosamine in Bacillus subtilis
A technology of Bacillus subtilis and recombinant bacteria, which is applied in the field of genetic engineering, can solve the problems of metabolic overflow and NADH consumption, and achieve the effect of simple construction method, reduction of NADH, and balance of intracellular reducing power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Construction of Bacillus subtilis BSGNKAP3
[0049] Bacillus subtilis BSGNKAP2 for B. subtilis 168ΔnagPΔgamPΔgamAΔnagAΔnagBΔldhΔptaΔgl cKΔpckAΔpyk P 43 -glmS P 43 - pycA::lox72, episomal expression of the GNA1 gene from the pP43NMK-GNA1 plasmid. Then, on this basis, the gene balpycA encoding pyruvate carboxylase BalpycA (NCBI-ProteinID: AAS42897) of Bacillus cereus was integrated at the malS site of the Bacillus subtilis genome, and was verified by bleomycin resistance plate screening and colony PCR , Sequencing, confirming that the integration was successful to obtain the recombinant Bacillus subtilis BSGNKAP3.
Embodiment 2
[0050] Example 2: Construction of Bacillus subtilis BSGNKAP4
[0051] Bacillus subtilis BSGNKAP3 was used as the host, and the pP43NMK-GNA1 plasmid expressed the GNA1 gene freely, and then integrated glyceraldehyde-3-phosphate ferredoxin dehydrogenase (NCBI-ProteinID: CAF30501 ) encoding gene gor, through bleomycin resistance plate screening, colony PCR verification, and sequencing, it was confirmed that the integration was successful and the recombinant Bacillus subtilis BSGNKAP4 was obtained.
Embodiment 3
[0052] Example 3: Construction of recombinant Bacillus subtilis BSGNKAP5
[0053] Using BSGNKAP4 as the host, the GNA1 gene was freely expressed with the pP43NMK-GNA1 plasmid, and then integrated isocitrate NAD at the ywkA site of the Bacillus subtilis genome + Dehydrogenase (NCBI-Protein ID: AKC61181) encoding gene icd was successfully integrated to obtain recombinant Bacillus subtilis BSGNKAP5 through bleomycin resistance plate screening, colony PCR verification, and sequencing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com