Preparation method of fluorene block thiophene cathode interface layer containing silane terminal group
A cathode interface layer and terminal group technology, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problem of poor morphology of the upper active layer, large interface barrier between the active layer and the electrode, and poor mechanical properties of the interface layer and other problems, to achieve the effect of improving the morphology, improving the mechanical properties, and improving the film-forming property
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The present invention will be further described below in conjunction with the accompanying drawings.
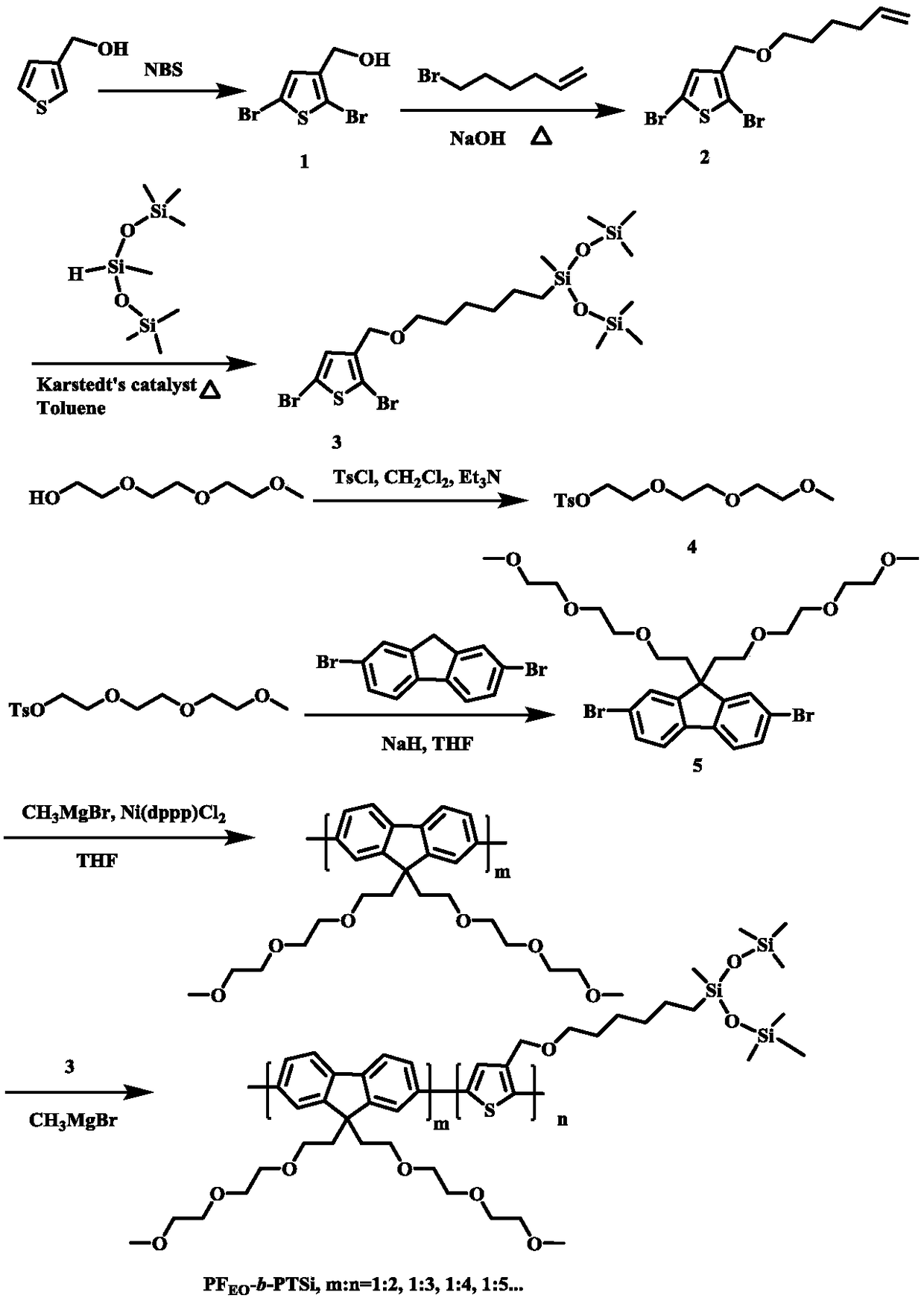
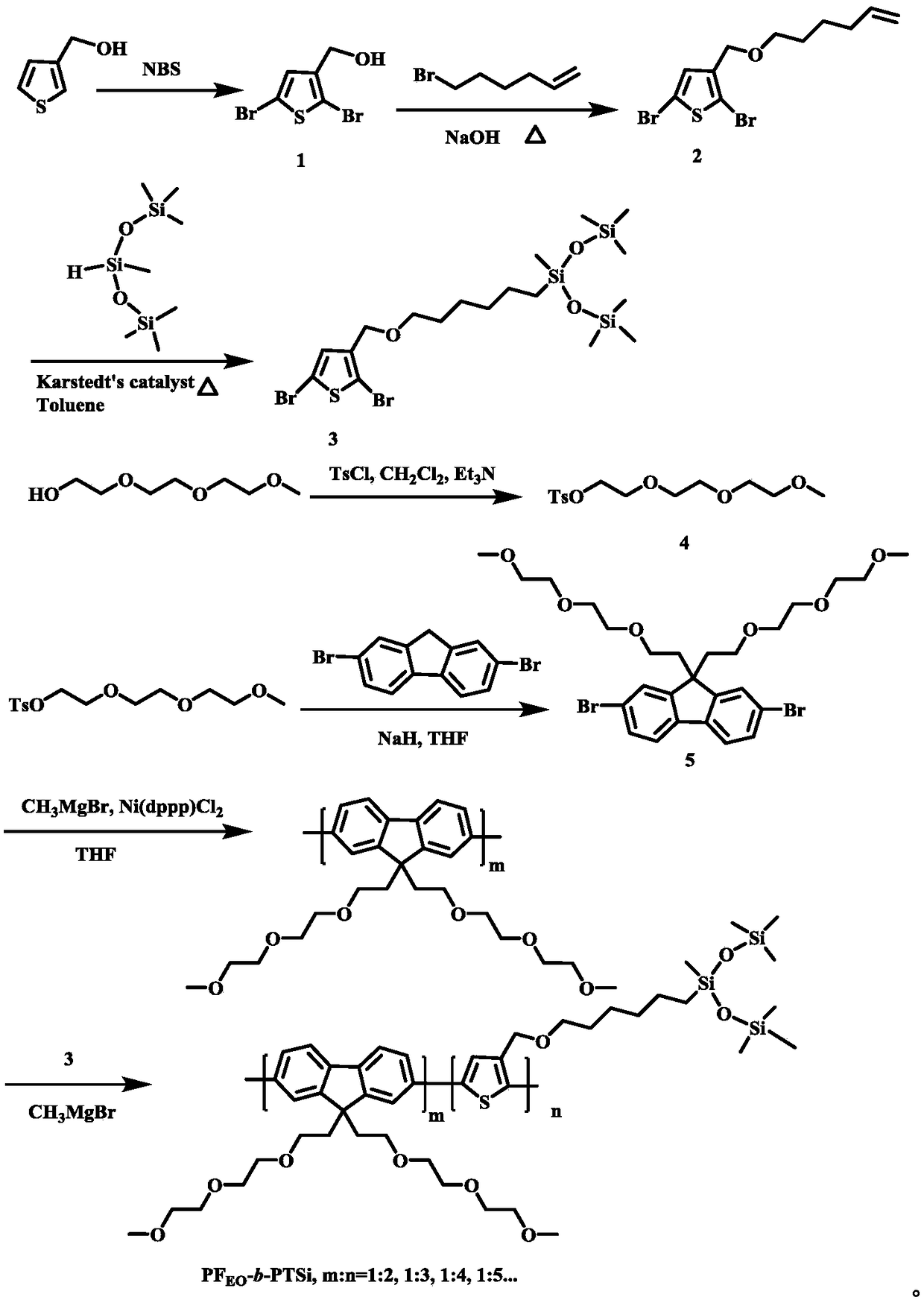
[0018] The reaction equation of the present invention is attached figure 2 , the specific reaction steps are as follows:
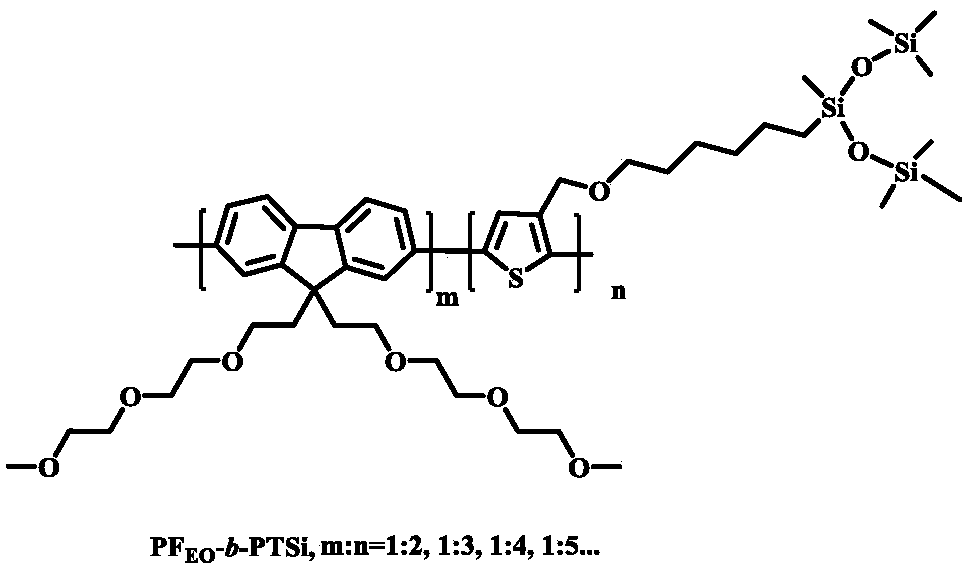
[0019] Fluorene block thiophene PF with silane terminal groups EO - b -Synthetic steps of PTSi:
[0020] (1) Synthesis of compound 1: Add 2.5 g, 22.0 mmol 3-thiophene methanol and 60 mL anhydrous tetrahydrofuran solution in a dry 250 mL round-bottom flask, then add 7.9 g, 44.0 mmol NBS in batches, and react overnight in the dark . Remove the NBS remaining in the reaction by filtration, remove the tetrahydrofuran solvent by a rotary evaporator, extract the product with 200 mL of ether and water, and wash with 1M sodium hydroxide solution, combine the organic layer products, and spin dry the obtained crude product with n-hexane: acetic acid The volume ratio of ethyl ester was 3:1, purified by column, and dried with anhydrous magnesium sulfate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com