A kind of paracetamol pharmaceutical composition preparation and preparation method thereof
A technology of acetaminophen and composition, which is applied in the field of acetaminophen pharmaceutical composition preparation and its preparation, can solve problems such as hairy tablet surface, lower pass rate, gastrointestinal tract damage, etc., and achieve stable physical and chemical properties , avoid irritation and damage, and the effect of uniform texture of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] According to one aspect of the present invention, a kind of preparation method of paracetamol pharmaceutical composition preparation comprises the steps:
[0030] (A) After paracetamol, crospovidone, povidone K30, methylparaben, ethylparaben and propylparaben are mixed uniformly, an aqueous binder solution is added for granulation;
[0031] (B) The stirring speed of granulation is between 200-400rpm, and the granulation time is 90-180s;
[0032] (C) The method of gradient temperature change is used for drying, and the temperature of each gradient is controlled between 20-40°C, and the temperature of the previous gradient and the subsequent gradient tend to alternate back and forth, and the difference between the two adjacent gradients 4-5°C, after drying, add alginic acid, calcium carbonate, and colloidal silicon dioxide to pre-mix for 3-5 minutes, then add magnesium stearate and mix for 3-5 minutes, press into tablets, and coat.
[0033] The invention provides a prepa...
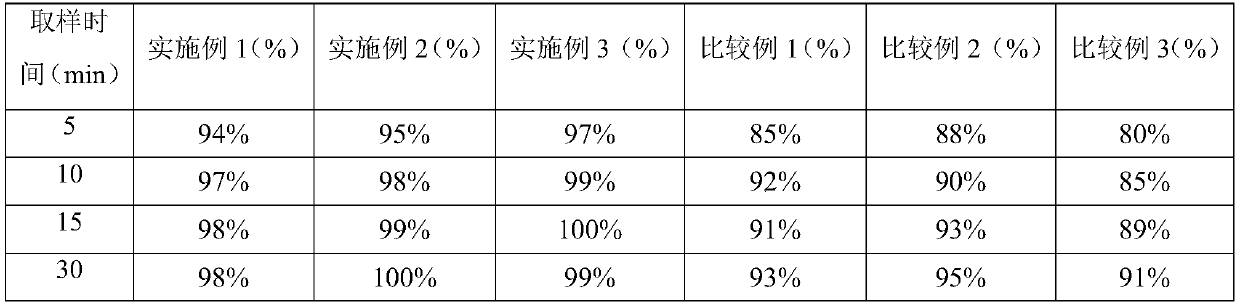
Embodiment 1
[0048] 1) According to production requirements, paracetamol, crospovidone, povidone K30, methylparaben, ethylparaben, propylparaben, alginic acid, calcium carbonate, colloidal silicon dioxide, hard These materials of magnesium fatty acid are weighed, and the order particle size of above-mentioned raw materials is controlled at more than 80 orders;
[0049] 2) Adhesive preparation: Weigh the pregelatinized starch of the adhesive prepared by half of the recipe, add an appropriate amount of boiling water to disperse and dissolve, and cool to room temperature to obtain an aqueous solution of pregelatinized starch;
[0050] 3) Mixed granulation: put acetaminophen, crospovidone, povidone K30, methylparaben, ethylparaben and propylparaben into a wet granulator and mix evenly, then add pre- For the gelatinized starch aqueous solution, start stirring and shearing, the stirring rate is 200rpm, and the granulation time is controlled within 90s;
[0051] 4) Put all the wet granules in th...
Embodiment 2
[0056] 1) According to production requirements, paracetamol, crospovidone, povidone K30, methylparaben, ethylparaben, propylparaben, alginic acid, calcium carbonate, colloidal silicon dioxide, hard These materials of magnesium fatty acid are weighed, and the order particle size of above-mentioned raw materials is controlled at more than 90 orders;
[0057] 2) Adhesive preparation: Weigh the pregelatinized starch of the adhesive prepared by half of the recipe, add an appropriate amount of boiling water to disperse and dissolve, and cool to room temperature to obtain an aqueous solution of pregelatinized starch with a concentration of 15% by mass;
[0058] 3) Mixed granulation: put acetaminophen, crospovidone, povidone K30, methylparaben, ethylparaben and propylparaben into a wet granulator and mix evenly, then add pre- For gelatinized starch aqueous solution, start stirring and shearing, the stirring rate is 400pm, and the granulation time is controlled within 180s;
[0059] 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com