Aluminum source for electrolytic preparation of aluminum-containing aluminum alloy, preparation method thereof and method for preparing aluminum-containing alloy through aluminum source
An aluminum alloy and aluminum source technology, applied in the field of metallurgy and materials, can solve the problems of unstable aluminum element in aluminum alloy, unstable aluminum content in aluminum alloy, difficult to control aluminum content, etc., to achieve stable content and reduce storage. Requirements for conditions and transportation conditions, the effect of improving input stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
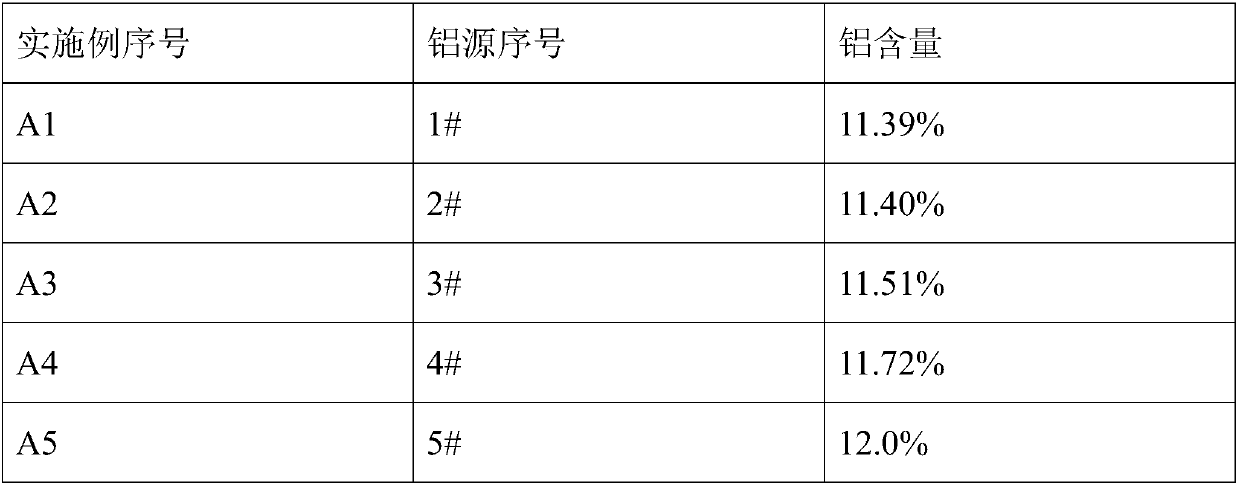
[0103] An aluminum source for electrolytic preparation of aluminum alloys, prepared by the following method:
[0104] Mixed with anhydrous AlCl with a water content of 0.5% 3 12.1 mol and 0.5 mol of NaCl with a water content of 1.5% were used to obtain an aluminum-containing mixture, and the obtained aluminum-containing mixture was heated to 105° C., kept for 30 minutes, and cooled to room temperature to obtain 1# aluminum source. The molar ratio of aluminum element to sodium element in 1# aluminum source is 24.0.
preparation example 2
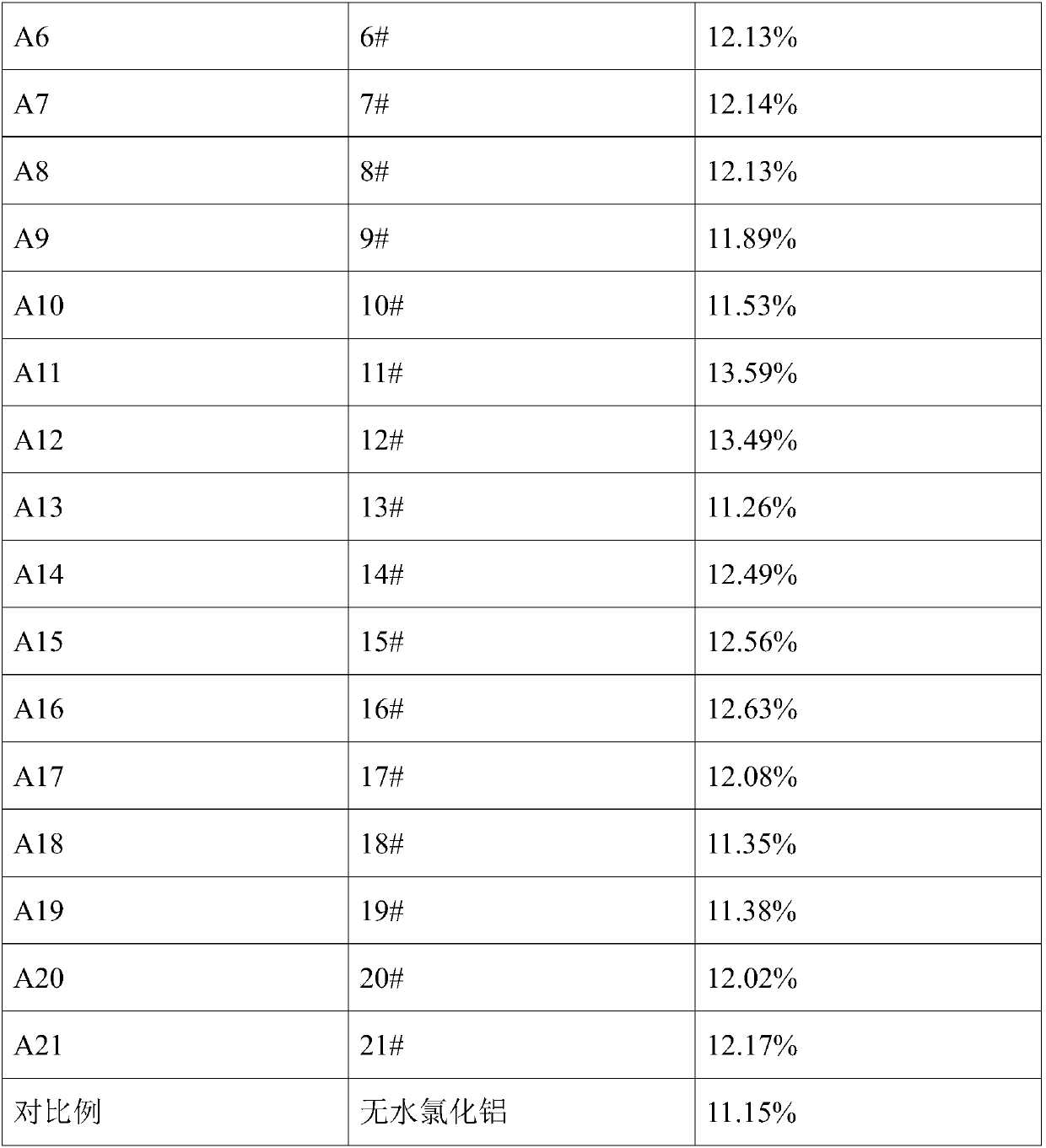
[0106] An aluminum source for the electrolytic preparation of aluminum alloys, the difference from Preparation Example 1 is that the aluminum-containing mixture is heated to 108.7°C, and liquid begins to appear, without heat preservation, and then cooled to room temperature to obtain 2# aluminum source . The molar ratio of aluminum element to sodium element in 2# aluminum source is 24.0.
preparation example 3
[0108] An aluminum source for electrolytic preparation of aluminum alloy, the difference from Preparation Example 1 is that the aluminum-containing mixture is heated to 108.7°C, kept for 2 minutes, and then cooled to room temperature to obtain 3# aluminum source. The molar ratio of aluminum element to sodium element in 3# aluminum source is 24.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com