Method for synthesizing biphenyl carboxylic acid compounds by suzuki coupling reaction
A technology of coupling reaction and biphenyl carboxylic acid, applied in the field of organic chemical synthesis, can solve the problems of harsh reaction conditions, unfriendly environment, and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
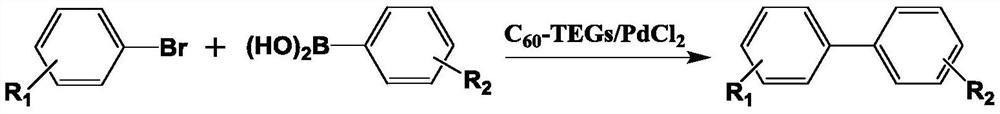
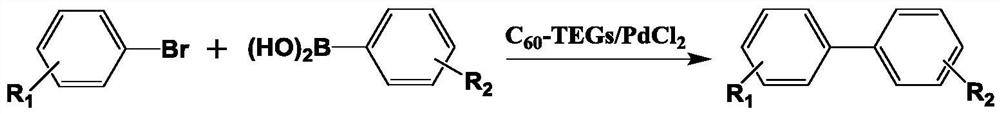
[0021] The present invention utilizes Suzuki coupling reaction to synthesize the method for biphenyl carboxylic acid compound, uses brominated aromatic hydrocarbon and aryl boronic acid as raw material, uses water-soluble fullerene nano-palladium as catalyst, and described Suzuki coupling reaction equation is as follows:
[0022]
[0023] In the formula, R 1 , R 2 Represents the substituents at different positions, which can be electron-withdrawing or electron-donating substituents, or single or multiple substitutions, R 1 , R 2 Can be the same or different groups; the reaction conditions are as follows:
[0024] The molar ratio of bromoarene to arylboronic acid is 1.0:1.0-1.5; the reaction temperature is 0-75°C; the reaction time is 2-10h; the base used in the reaction is K 2 CO 3 、Na 2 CO 3 、Cs 2 CO 3 , KF, K 3 PO 4 any one or more of them. It is preferable to use K 2 CO 3 .
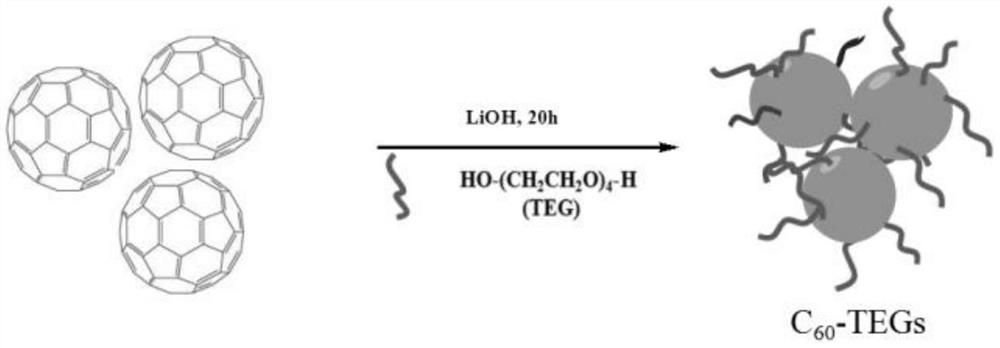
[0025] Below is the preparation method of the catalyst used in the present inventi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com