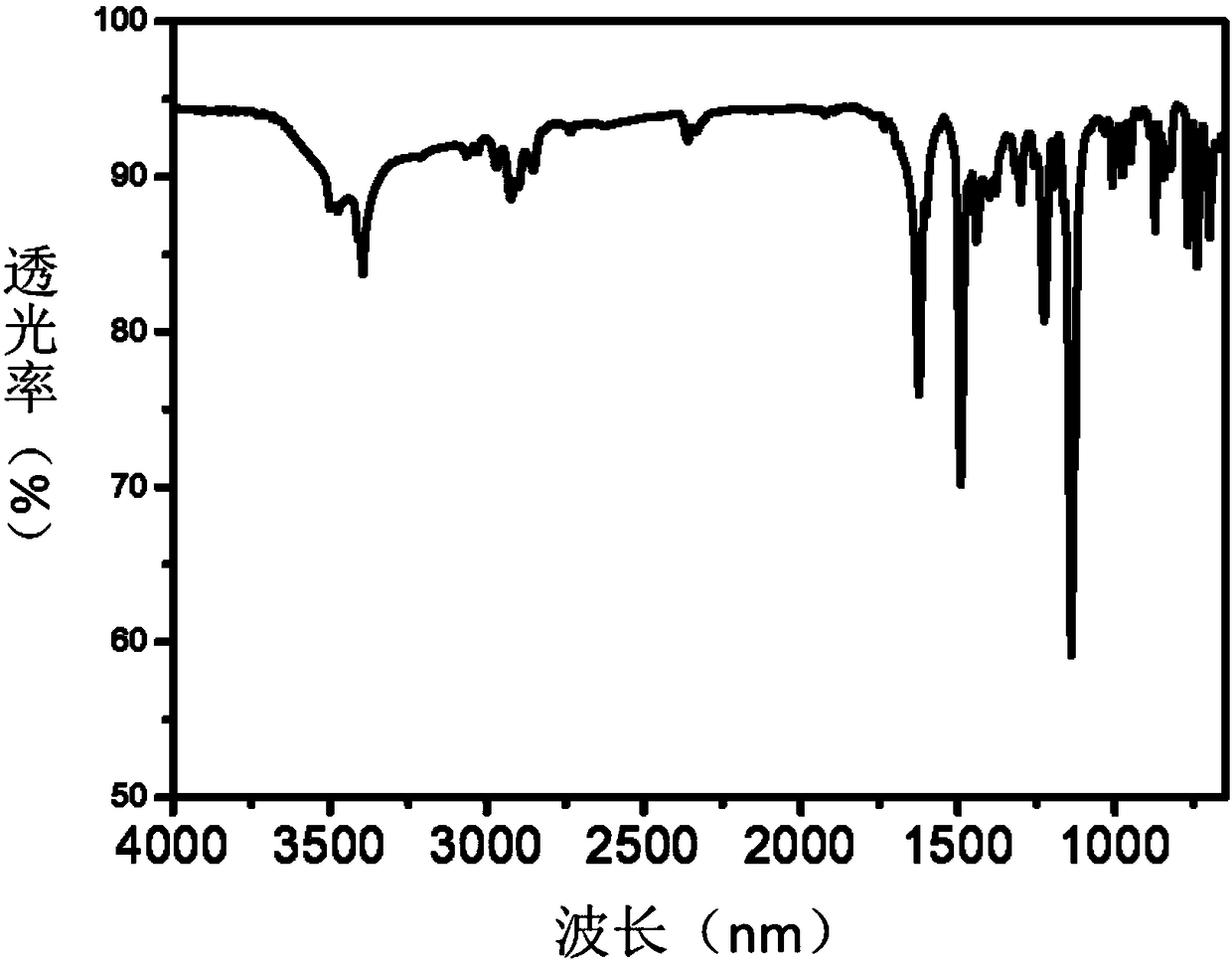
Aromatic diamine and preparation method thereof
An aromatic diamine and biphenyl technology, applied in the field of aromatic diamine and its preparation, can solve the problems of high price, difficult preparation of aromatic diamine, lowering the thermal decomposition temperature of polyimide, etc., and achieves cheap raw materials and thermal stability. Good, easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] In the preparation of the aromatic diamine of the present application, the structure of the biphenyl compound is a key component.
[0062] In one embodiment of one aspect of the present application, the biphenyl compound comprises the following structural formula:
[0063]
[0064] Wherein, the biphenyl compound is prepared by reacting trifluoroacetic anhydride and biphenyl, and the preparation method comprises:
[0065] Under the condition of ice bath, trifluoroacetic anhydride, biphenyl, catalyst anhydrous aluminum trichloride and solvent dichloromethane were reacted at room temperature, and the reaction crude product was poured into hydrochloric acid solution, and then extracted with dichloromethane. The obtained organic phase was successively washed with sodium bicarbonate solution and saturated brine, dried with anhydrous magnesium sulfate, and spin-dried to obtain the biphenyl product.
[0066] Concrete reaction formula is as follows:
[0067]
[0068] Whe...
Embodiment 1
[0090] In this embodiment, it first involves the preparation of a biphenyl compound having the following structural formula:
[0091]
[0092] The preparation method of the biphenyl compound is as follows: disperse 13.3g (100mmol) of anhydrous aluminum trichloride in 125mL of dichloromethane in a 250mL one-necked flask under ice bath conditions. After stirring for 0.5 h, slowly add 21 g (14.1 mL, 100 mmol) of trifluoroacetic anhydride in 40 mL of methylene chloride solution dropwise into the dispersion within 1 h, and after stirring for 15 min, dissolve 7.7 g (50 mmol) of biphenyl in 40 mL of Dichloromethane was slowly added to the reaction solution within 1 h. Stir at room temperature, spot plate detection reaction until the end of the reaction. After completion, at room temperature, the reaction solution was slowly poured into 550 mL of hydrochloric acid solution (250 mL of concentrated hydrochloric acid, 150 mL of water, 150 g of ice), extracted with dichloromethane, an...
Embodiment 2
[0117] In this embodiment, first prepare the biphenyl compound with following structural formula:
[0118]
[0119] The preparation method of this biphenyl compound is: with 4.625g (25mmol) p-bromobenzaldehyde, 9.495g (50mmol) p-trifluoromethylphenylboronic acid, 1.445g (1.25mmol) tetrakis (triphenylphosphine) palladium, 13.8g (100mmol) Potassium carbonate, 100mL ethylene glycol dimethyl ether and 15mL water were mixed, nitrogen was pumped out three times, and reacted at 110°C for 24h. After the reaction was completed, it was extracted with dichloromethane, the organic phase was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and separated by column chromatography to obtain the product (3.878g), with a yield of about 62%.
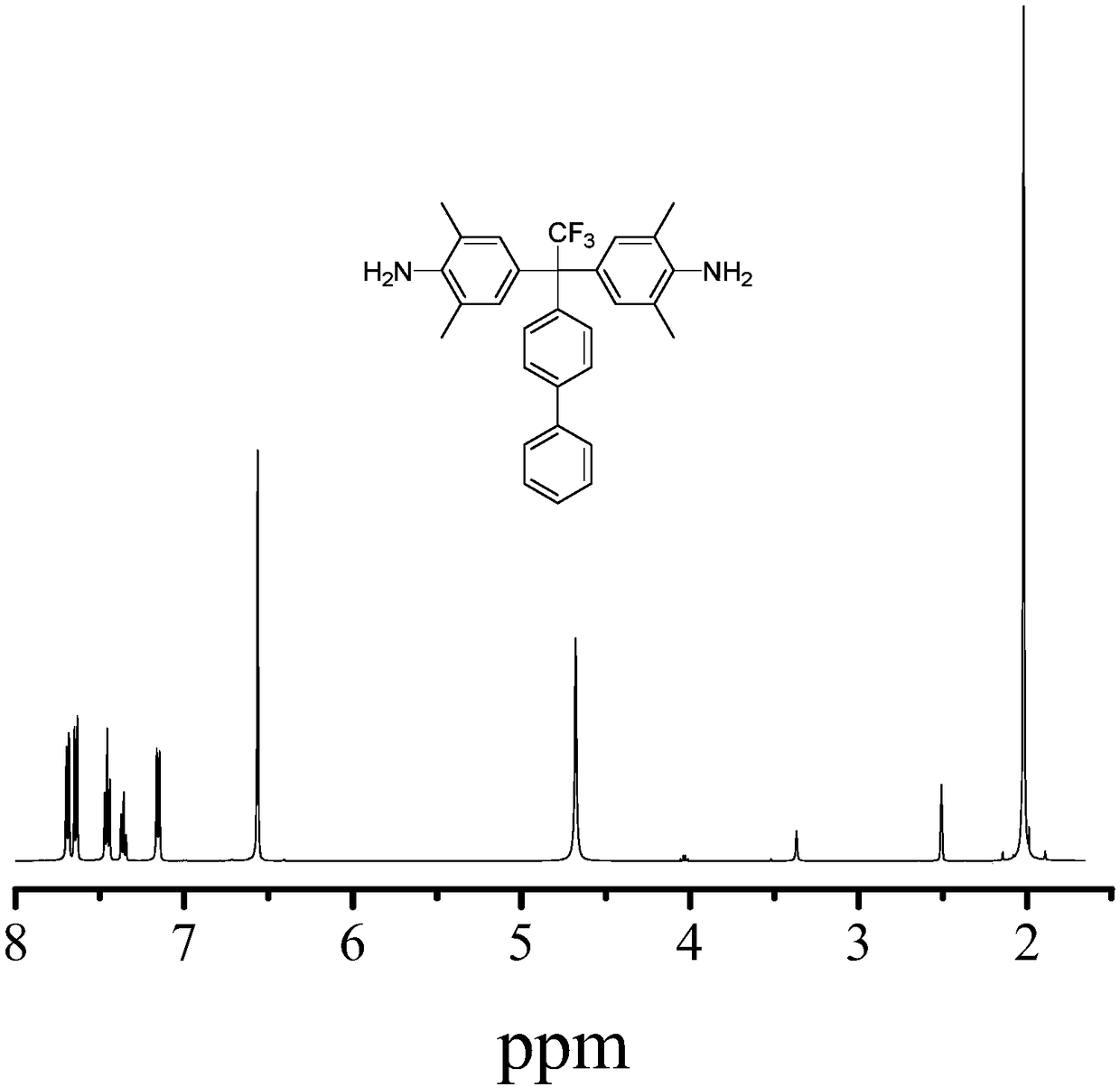
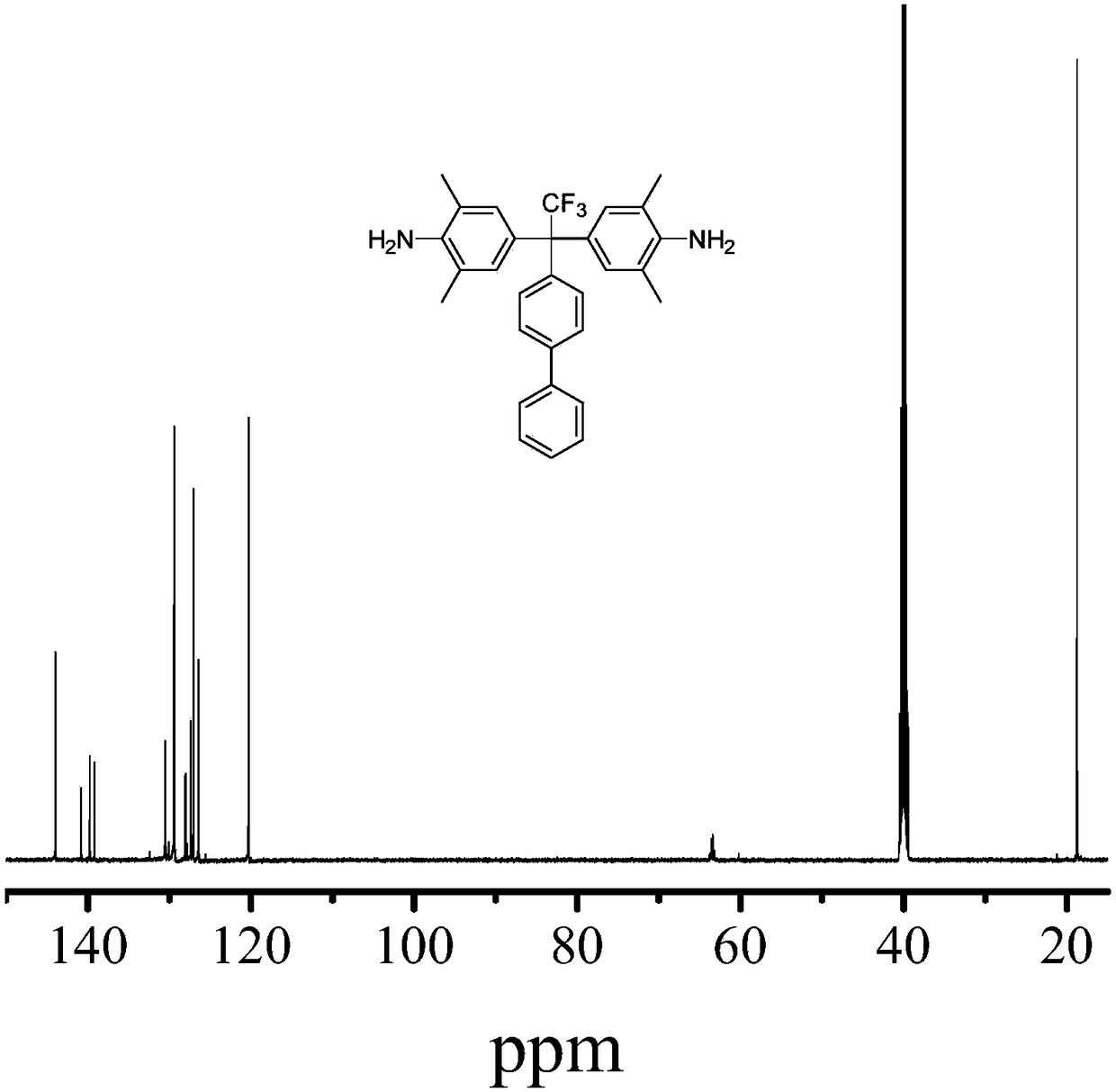
[0120] This biphenyl compound is carried out NMR test, concrete result is as follows:
[0121] 1 H NMR :
[0122] Proton NMR data were obtained with 400MHz AVANCE III HD (Bruker BioSpin Corp., Germany).
[0123] produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com