Medicine composition for preventing or treating pyohemia
A composition and sepsis technology, applied in the field of biomedicine, can solve the problems of systemic inflammatory response and circulatory metabolic state changes, inability to simulate clinical characteristics well, rapid death of animals, etc., and achieve the effect of inhibiting organ failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The test method is as follows:
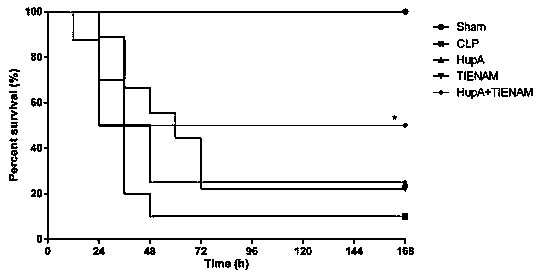
[0022] Effect of combination of huperzine A HupA and antibiotic TIENAM on the survival rate of CLP sepsis mice
[0023] 100 mice were randomly divided into 5 groups, sham operation control group (Sham group), normal saline control group (CLP group), HupA treatment group (0.1mg / kg·d), TIENAM group (50mg / kg·d) Combined with HupA+TIENAM group, 20 mice in each group, all mice were fasted for 12 hours before operation, and had free access to water. The blank control group received no treatment. The CLP sepsis mouse model was prepared according to the instructions in the literature. After laparotomy in the sham-operated control group, the cecum was freed, ligated, and no perforation was performed, and then the abdominal cavity was returned, and the abdomen was closed and sutured. At the end of the operation, 120 mice were subcutaneously injected with 1 ml of 37°C preheated normal saline on the back for fluid resuscitation; intraperitoneal in...
Embodiment 2
[0028] Detection of expression levels of inflammatory factors in serum
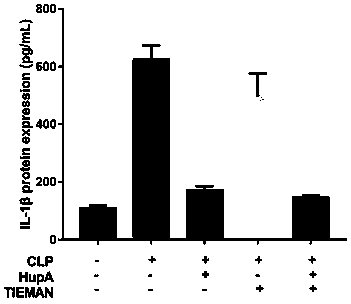
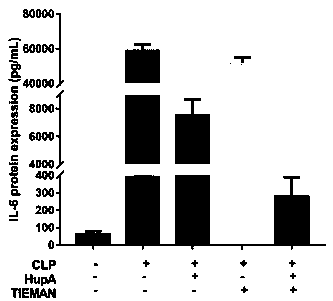
[0029] According to the operation steps of the ELISA kit manual, the expression levels of inflammatory factors IL-6, TNF-α, and IL-1β in peripheral blood serum were detected.
[0030] The test results are as follows:
[0031] Effects of HupA combined with TIENAM on peripheral blood cytokine levels in mice
[0032] As shown in Figure 2, 48 hours after operation, the levels of inflammatory factors such as TNF-α, IL-1β, and IL-6 in the serum of mice in the sham operation group were lower; were significantly increased, and the difference was significant ( P ), with statistical significance; TNF-ɑ, IL-1β, and IL-6 levels in the serum of mice in HupA group and HupA+TIENAM group were significantly lower than those in the CLP sepsis mouse model, and the difference was statistically significant ( P<0.01 ).
Embodiment 3
[0034] Histopathological examination
[0035] Forty-eight hours after the operation, they were killed by dislocation of the neck, and the lungs and kidneys of the mice were collected, fixed with 4% paraformaldehyde immediately, and numbered, dehydrated, transparent, soaked in wax, embedded, sliced, and dewaxed on baked slices according to the conventional methods of pathological examination. HE staining, tissue lesions were observed under an optical microscope.
[0036] The test results are as follows:
[0037] Observation on the effect of HupA combined with TIENAM on the histopathology of CLP sepsis mice
[0038] Grouped according to the experiment, CLP sepsis mice were sacrificed by neck dislocation 48 hours after administration, and the main organs of each group, such as lung, liver, spleen, and kidney, were collected for HE staining, and their pathological changes were observed under a microscope.
[0039] (1) if image 3 As shown, the mice were randomly divided into 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com