Preparation method and application of CuNi-based catalyst
A technology of catalysts and additives, applied in the field of preparation of CuNi-based catalysts, can solve problems such as difficulty in application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
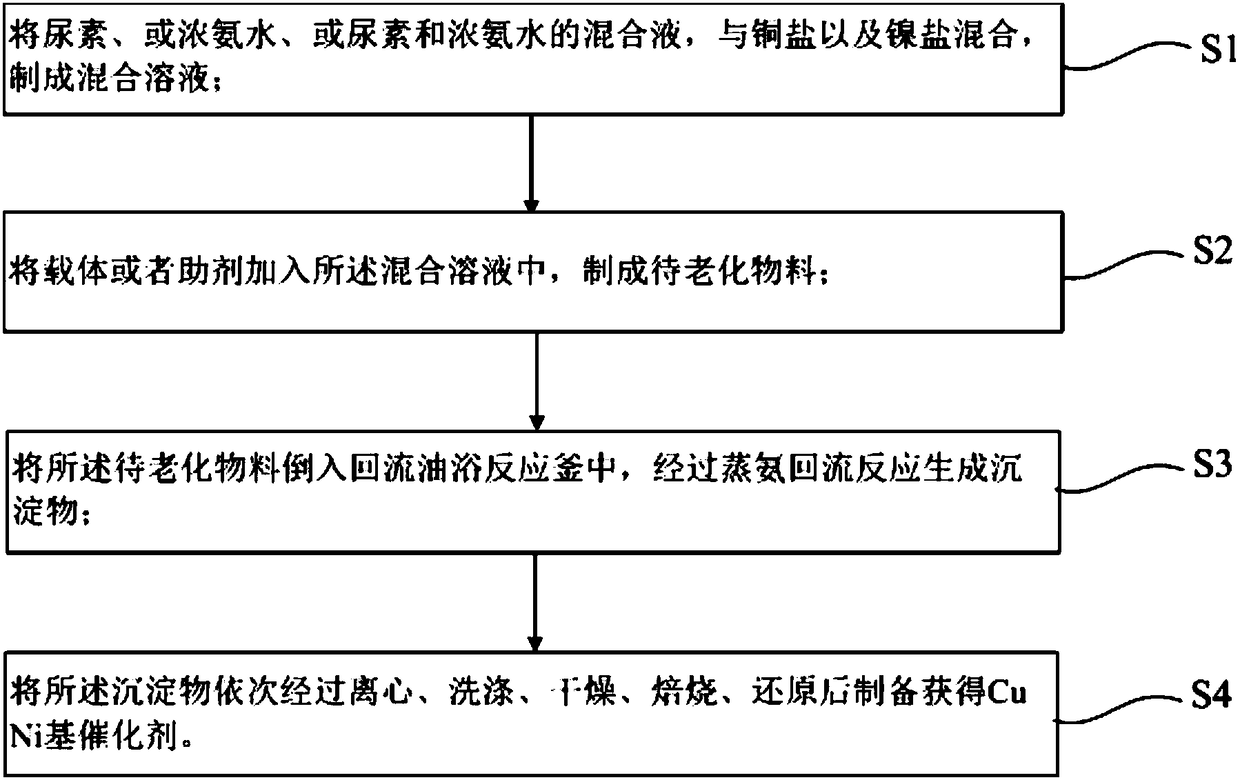
[0029] The present invention provides a kind of preparation method and application of CuNi base catalyst, such as figure 1 Shown, described preparation method comprises at least:
[0030] S1, mixing urea, or concentrated ammonia water, or a mixture of urea and concentrated ammonia water, with copper salt and nickel salt to make a mixed solution;
[0031] S2, adding a carrier or an auxiliary agent into the mixed solution to make a material to be aged;
[0032] S3, pouring the material to be aged into a reflux oil bath reactor, and generating a precipitate through ammonia distillation reflux reaction;
[0033] S4, preparing the CuNi-based catalyst by successively centrifuging, washing, drying, calcining and reducing the precipitate.
[0034] In addition, the present invention also provides the application of a CuNi-based catalyst for CO 2 and HN(CH 3 ) 2 In the reaction of hydrogenation reaction to synthesize DMF, the reaction adopts a high-pressure reactor, and the reaction ...
Embodiment 1
[0037] Take an appropriate amount of copper nitrate, nickel nitrate, 40% Wt% silica sol and concentrated ammonia water according to Cu:Ni=1:1, SiO 2 : Ni = 10: 1, concentrated ammonia water: (Cu+Ni) = 15: 1 (molar ratio) to prepare a 1M mixed material. Put the mixed material in a three-necked flask with a condensing reflux tube, under the heating of an oil bath, reflux and age at 80°C for 16 hours, centrifuge the reaction solution, wash, and dry to obtain a solid precipitate, and heat up at 1°C / min The rate was raised to 500°C and fired for 6 hours to obtain a metal oxide precursor. Weigh 0.20g of the metal oxide precursor and put it into the reduction tube. 2 Reduction under the atmosphere, the reduction conditions are: increase the temperature at a rate of 1°C / min to 500°C, reduce for 3h, H 2 Flow velocity 20ml / min, obtain CuNi base catalyst after reduction, wherein this CuNi base catalyst comprises CuOWt%: 28.5%, NiOWt%: 24.3%, SiO 2 Wt%: 47.2%.
[0038] Put 0.2g of the...
Embodiment 2
[0040] Take appropriate amount of copper nitrate, nickel nitrate, 40%wt% silica sol and urea according to Cu:Ni=3:1, SiO 2 : Ni=10:1, urea: (Cu+Ni)=8:1 (molar ratio) prepared into a 1.5M mixed material. Put the mixed material in a three-necked flask with a condensing reflux tube, under the heating of an oil bath, reflux and age at 80°C for 16 hours, centrifuge the reaction solution, wash, and dry to obtain a solid precipitate, and heat up at 1°C / min Raise the rate to 600°C, and bake for 3 hours to obtain a metal oxide precursor. Weigh 0.20g of the metal oxide precursor and put it into the reduction tube. 2 Reduction under the atmosphere, the reduction conditions are: increase the temperature at a rate of 1°C / min to 600°C, reduce for 3h, H 2 Flow rate 20ml / min, obtain CuNi base catalyst after reduction, wherein this CuNi base catalyst comprises CuOWt%: 30.5%, NiOWt%: 9.8%, SiO 2 Wt%: 59.7%.
[0041] Put 0.2 g of the above-mentioned reduced CuNi-based catalyst into the autoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com