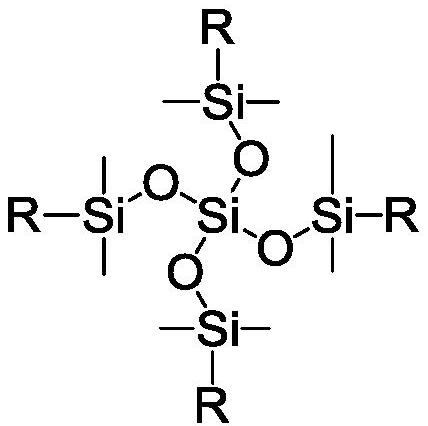
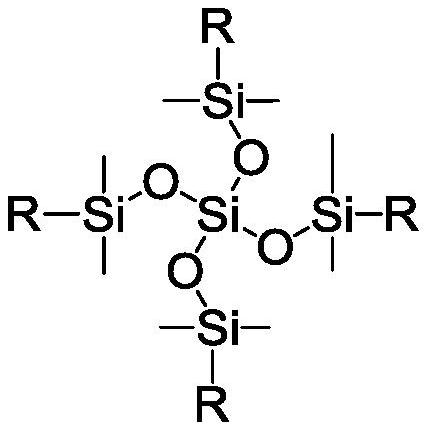
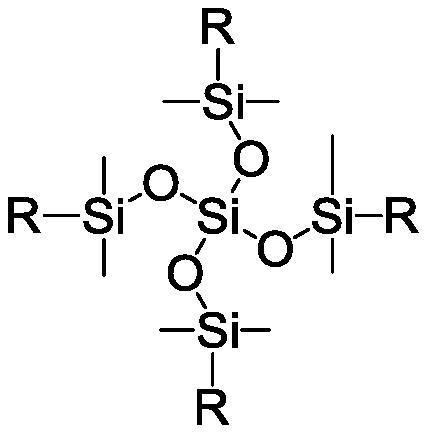
Benzocyclobutene-functionalized tetrakis(dimethylsiloxy)silane, its preparation method and its resin preparation method
A benzocyclobutene functional, dimethylsiloxy technology, applied in chemical instruments and methods, organic chemistry, silicon-organic compounds, etc., can solve the problems of low dielectric properties, difficult processing, poor film-forming properties, etc. , to achieve the effect of low dielectric properties, easy operation and moderate curing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] Another aspect of the present invention provides a method for preparing a benzocyclobutyl functionalized tetra (dimethylsiloxy) silane. In an exemplary embodiment of the preparation method of the preparation method of the benzocybutylene functionalized four (dimethylsiloxy) silane of the present invention, the preparation method can include:
[0027] Step S01, synthesized 4-dimethylvinyl silica and cyclobutylene.
[0028] In this example, the method of synthesizing 4-dimethylvinyl silicide (referred to as 4-DMVSBCB) can include:
[0029] In step S0101, the magnesium strip is added to the reactor, and tetrahydrofuran and iodine particles are added to the reactor under an inert atmosphere condition. The mixed solution obtained in tetrahydrofuran was then added to the mixed solution obtained in tetrahydrofuran. The mixed solution was added dropwise to the magnesium strip and iodine particles at room temperature, and the mixed solution was slowly added dropwise to the mixed sol...
example 1
[0072] A, synthesis of 4-dimethylvinyl silicate cyclobutylene (referred to as 4-DMVSBCB).
[0073] The magnetic stirrer and 2.92 g of magnesium strip were added to the reactor, and the vacuum filled nitrogen gas was repeated 3 times (ensuring oxygen in the system), and 5 ml of tetrahydrofuran and 0.010 g of iodine-activated magnesium strip were added to the reactor under nitrogen protection. 20 g of 4-bromobenzene was taptene was diluted in 36 ml of tetrahydrofuran to give a mixed solution. The addition of the drip mixed solution with magnesium strips and iodine were exposed to the magnesium strip and iodine, and the mixed solution was slowly added dropwise, and the mixed solution was continued. After the dropwise addition, the system was transferred to a 65 ° C oil bath reaction for 1 h. Then, the temperature dropped at a nitrogen atmosphere to room temperature, and 14 g of dimethylacetin-based chlorosilane was removed from 30 ml of tetrahydrofuran to give a mixed liquid, and the...
example 2
[0084] A, synthesis of 4-dimethylvinyl silicate cyclobutylene (referred to as 4-DMVSBCB).
[0085] The magnetic stirrer and 3.10 g of magnesium strip were added to the reactor, and the vacuum filled nitrogen gas was repeated 3 times (ensuring oxygen in the system), and 6 ml of tetrahydrofuran and 0.010 g of iodine-activated magnesium strip were added to the reactor under nitrogen protection. 20 g of 4-bromobenzene was taptene was diluted in 36 ml of tetrahydrofuran to give a mixed solution. The addition of the drip mixed solution with magnesium strips and iodine were exposed to the magnesium strip and iodine, and the mixed solution was slowly added dropwise, and the mixed solution was continued. After the dropwise addition, the system was transferred to a 70 ° C oil bath reaction for 2 h. Then the temperature dropped temperature to room temperature in a nitrogen atmosphere, and was diluted in 30 ml of tetrahydrofuran to give a mixture in 30 ml of tetrahydrofuran, and the mixture w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com