Naphthalimide-rhodamine-based compound and application thereof
A technology of naphthalimide and compound is applied in the field of compounds based on naphthalimide-rhodamine, and achieves the effects of rapid detection, easy post-processing and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
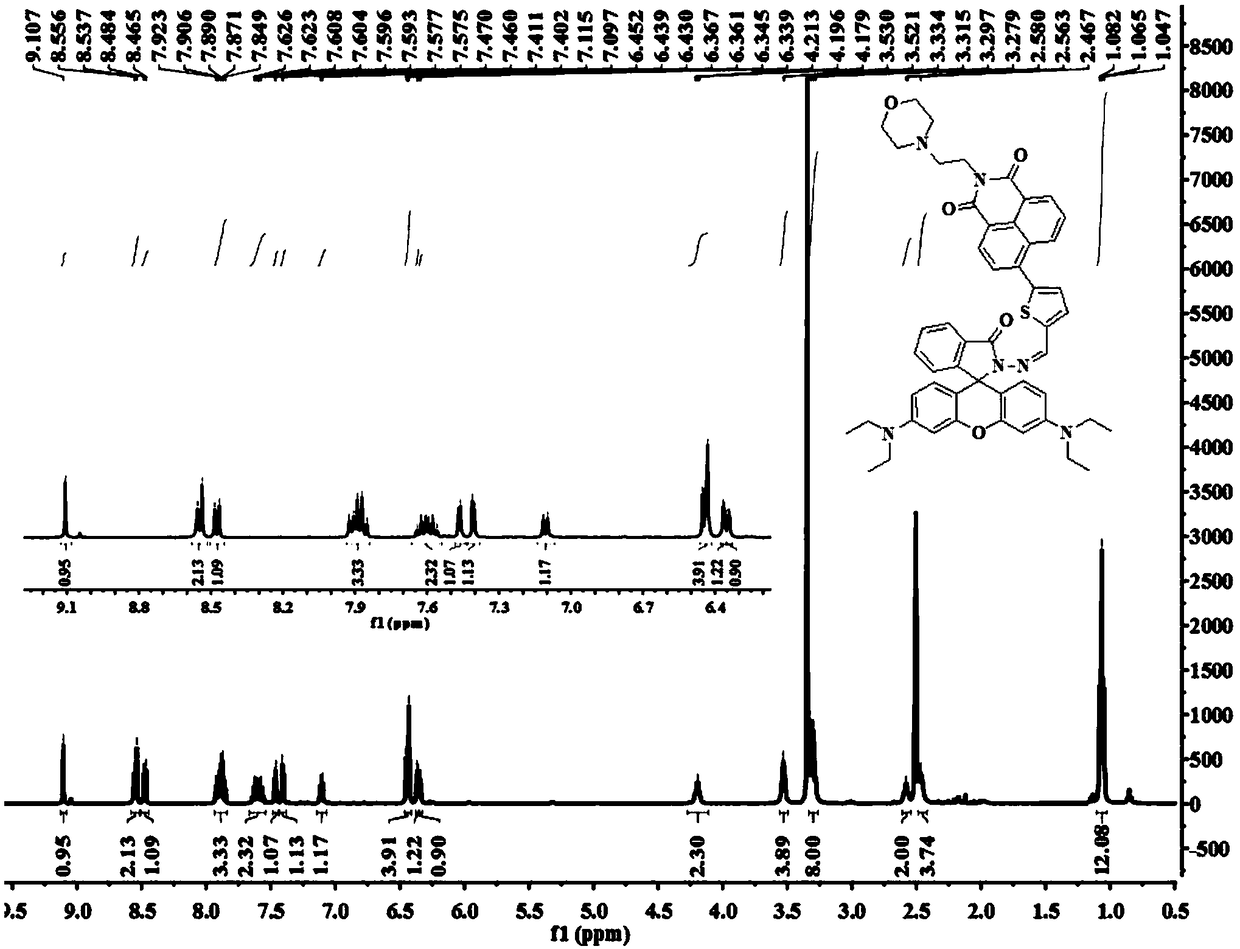
[0068] Preparation of target compound I-1
[0069]
[0070] (1) Synthesis of compound IV-1
[0071] 4-(2-Aminoethyl)morpholine (2.5g, 18mmol) and 4-bromo-1,8-naphthalic anhydride (2.5g, 9mmol) were dissolved in 50mL of ethanol, and the mixture was refluxed overnight, then cooled to At room temperature, the gray crude product was collected by filtration and then purified by column chromatography (eluent: dichloromethane) to obtain 3.07 g of off-white solid with a yield of 87%. 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.66 (d, J = 7.2, 1H), 8.58 (d, J = 8.4, 1H), 8.41 (d, J = 8.0, 1H), 8.05 (d, J = 8.0, 1H), 7.86(t, J=7.6,1H), 4.35(t, J=6.8,2H), 3.69(t, J=4.4,4H), 2.72(t, J=6.8,2H), 2.61(br,4H) .
[0072] The synthetic route is as follows:
[0073]
[0074] (2) Synthesis of compound Ⅵ-1
[0075] Add 2M K 2 CO 3 aqueous solution (10mL), add tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 ) (100mg, 0.08mmol), and the mixture was refluxed under nitrogen protection. ...
Embodiment 2
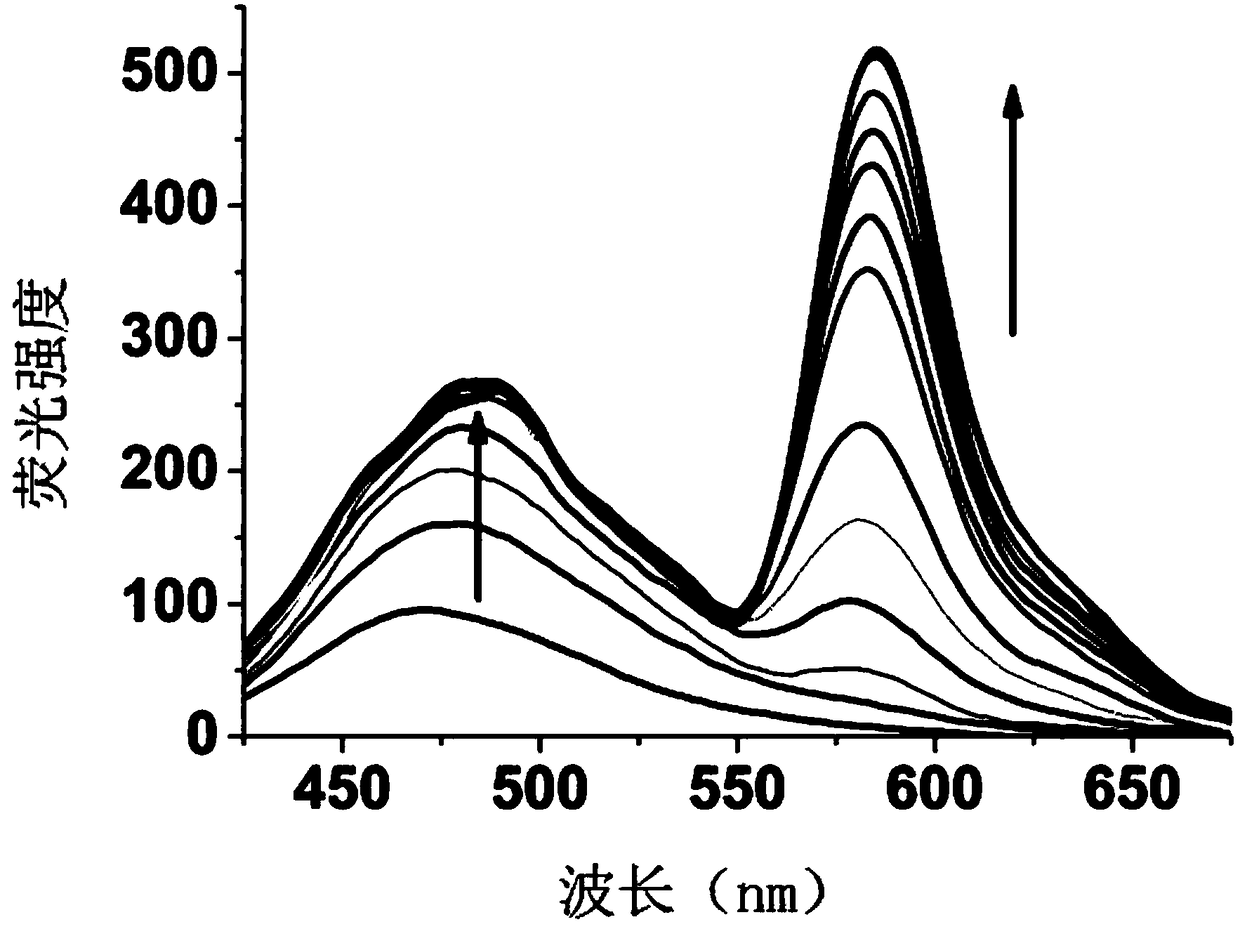
[0086] Compound I-1 changes in fluorescence spectrum with the increase of mercury ion addition:
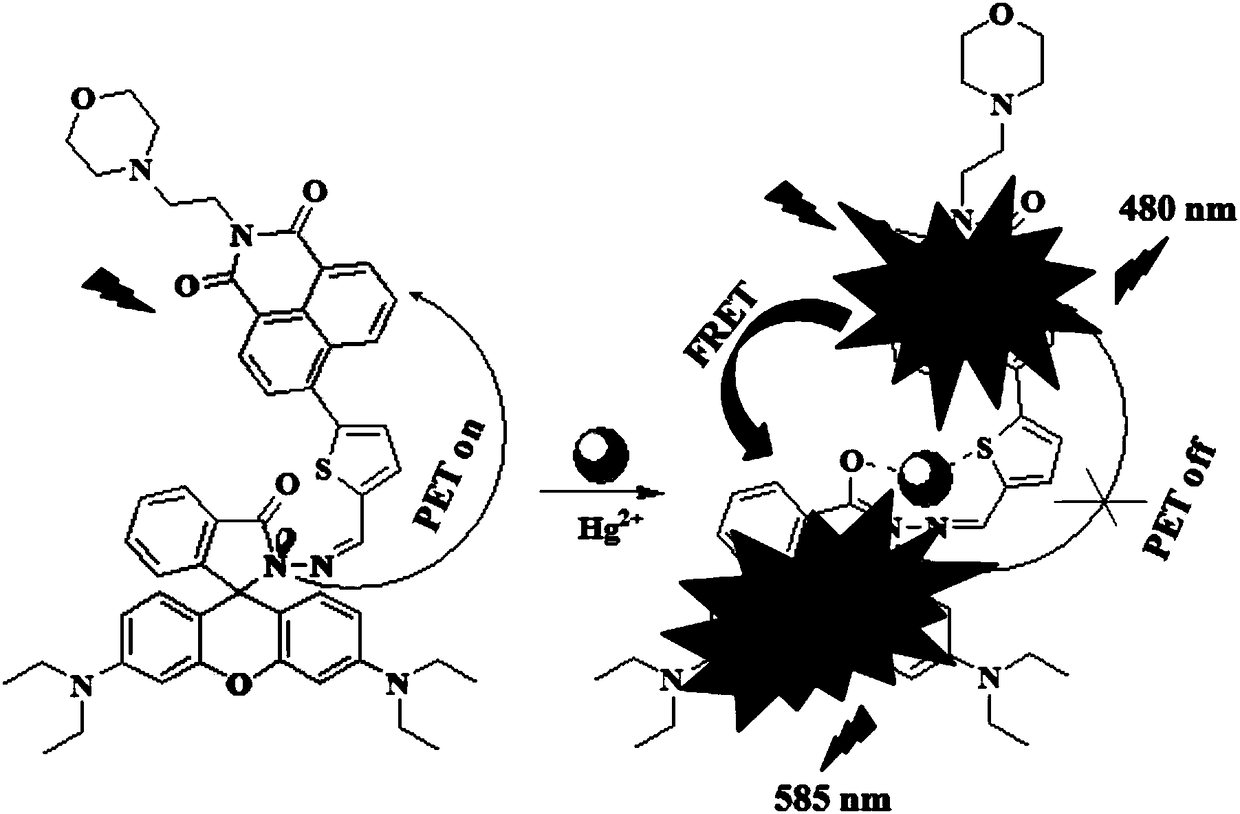
[0087] The compound I-1 prepared in Example 1 was dissolved in a solution of ethanol and deionized water, the volume ratio of ethanol and deionized water was 5:1, and a 20 μM test stock solution was prepared, and different equivalents (0-30eq) were added Mercury ion standard solution, with 400nm as excitation light, measure its fluorescence properties, fluorescence spectrum as image 3 as shown, image 3 It is the fluorescence spectrogram of adding different amounts of mercury ions in the compound I-1 prepared in Example 1, by image 3 It can be seen that with the addition of mercury ions, the fluorescence intensity of the naphthalimide part increases gradually, and slowly red shifts to 480nm. At the same time, the characteristic emission peak corresponding to the ring-opening of rhodamine appeared at 585nm, and gradually increased with the addition of mercury ions. This phenom...
Embodiment 3
[0089] Selectivity of Compound I-1 to Different Molecules or Ions
[0090] Take out 3mL respectively from the 20 μ M test stock solution in the embodiment 2 and add in the cuvette, add 30 times of equivalent competing molecular standard solutions, one of them adds the mercury ion standard solution of equimolar quantity, with 400nm as excitation light, detection solution Fluorescence emission spectrum changes, the results are as follows Figure 4 as shown, Figure 4 It is the selective fluorescence spectrogram of compound I-1 prepared in Example 1 to different ions. Depend on Figure 4 It can be seen that other metal ions have almost no effect on the fluorescence of compound I-1, but the addition of mercury ion solution significantly enhances the fluorescence of compound I-1 at 480nm and 585nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com