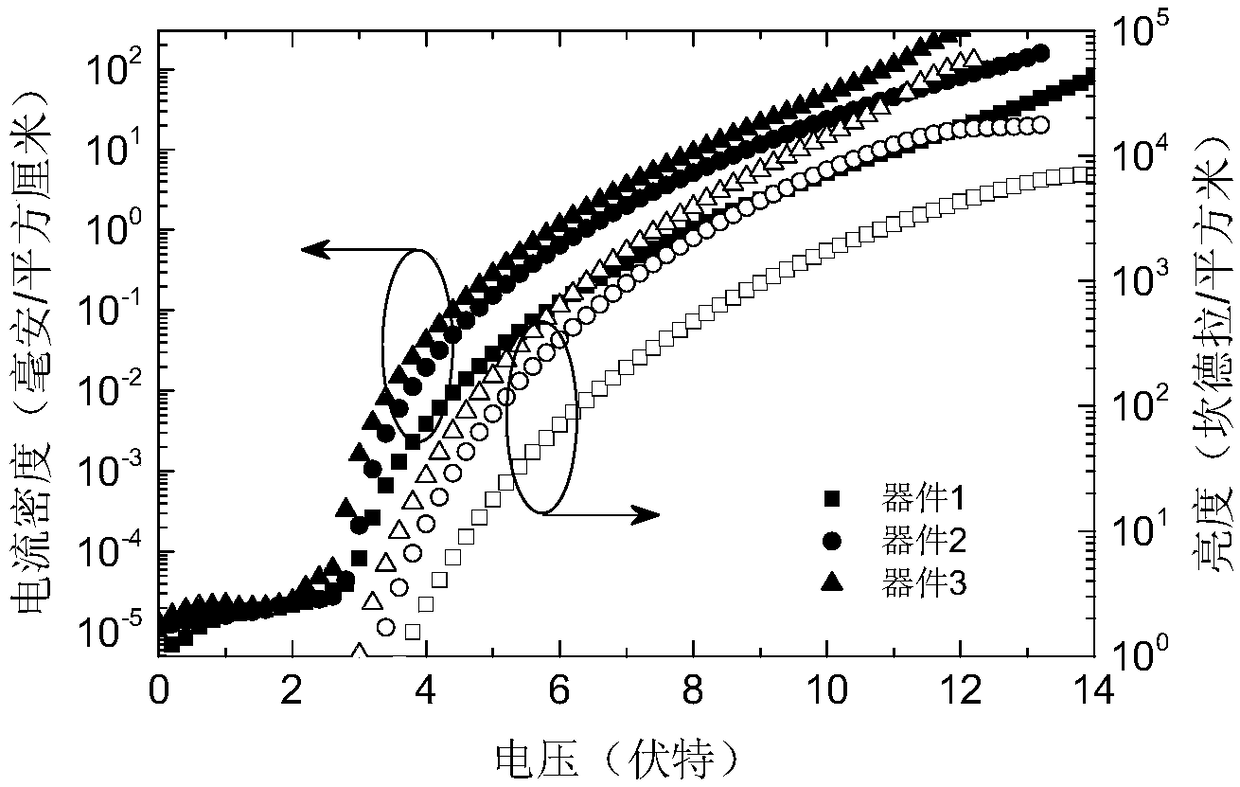
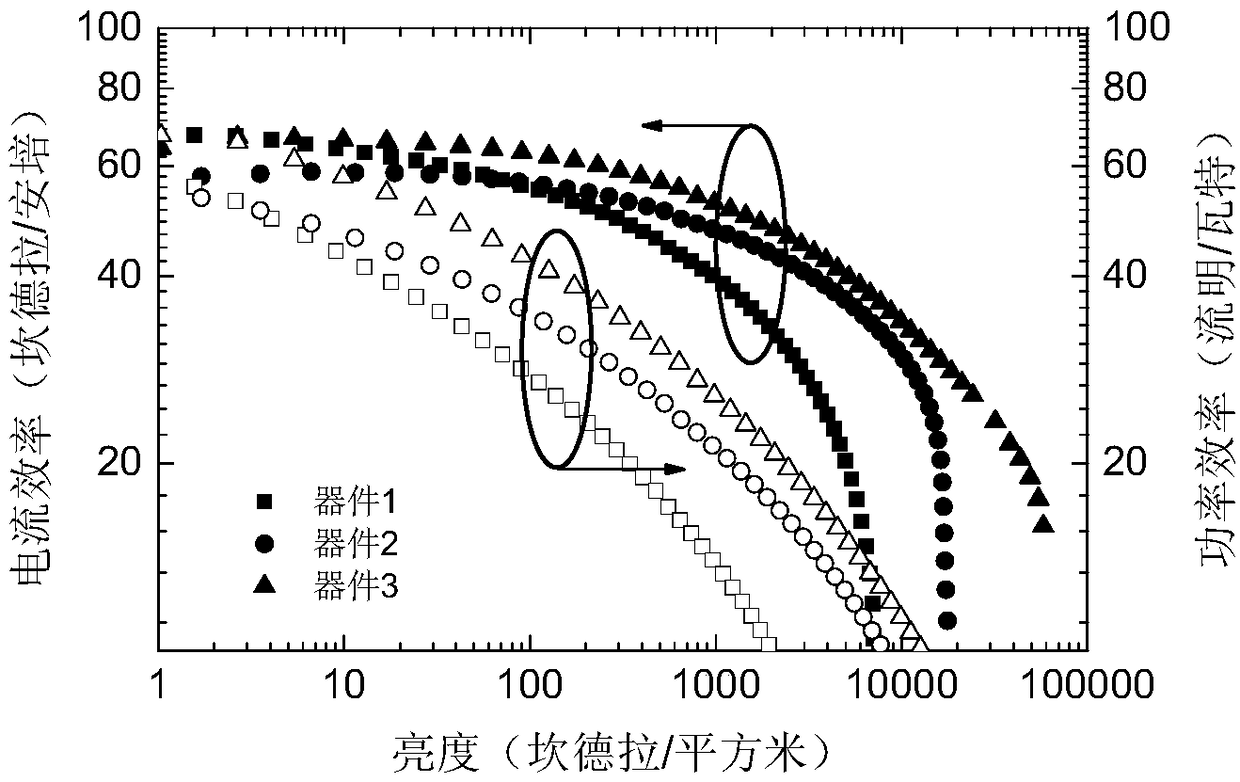
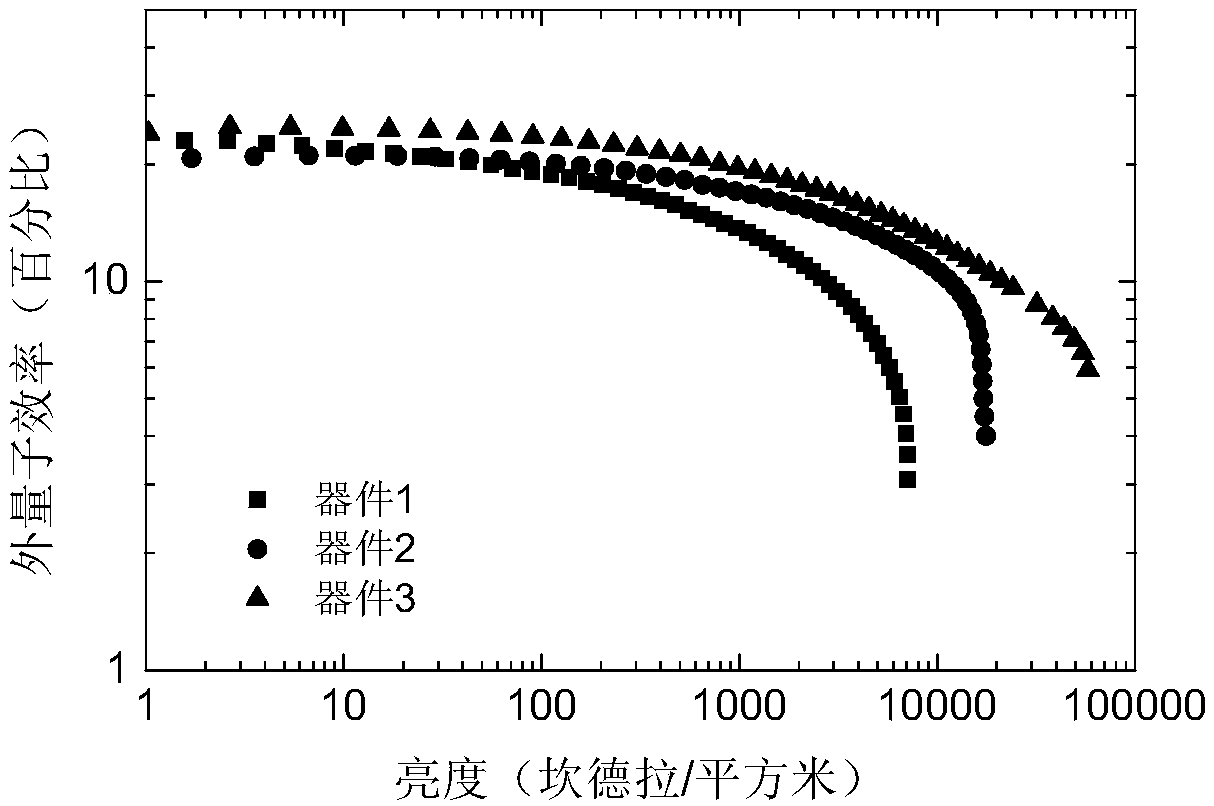
Organic micromolecule material based on 2,6-dimethyl-4-cyanophenyl receptor unit, preparation and application
An acceptor unit and small molecule technology, applied in the field of organic small molecule materials, to achieve the effect of easy purification, good reproducibility and low sublimation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of organic small molecule materials based on 2,6-dimethyl-4-benzonitrile acceptor unit with structural formula 1:
[0030] (1) Synthesis of intermediate product 1: under a nitrogen atmosphere, 183 mg of phenoxazine and 10 ml of bromobenzene were added to a 250 ml wide-mouth three-necked round-bottomed reaction flask, and dissolved in 70 ml of toluene solution. Add 5 grams of potassium carbonate subsequently, stir and ventilate for 15 minutes. Subsequently, a solution of 44.6 mg of palladium acetate and 0.73 mg of tri-tert-butylphosphine was added, stirred with aeration for 30 minutes, and then heated to 110 degrees Celsius. Reflux for 24 hours. After cooling down to stop the reaction, the liquid in the reaction system was removed by rotary evaporation to obtain the intermediate product 10-phenyl-10H-phenoxazine. Product Molecular Formula: C 18 h 13 NO; molecular weight: 259.31; elemental analysis results: C, 83.37; H, 5.05; N, 5.40; O, 6.17. The reactio...
Embodiment 2
[0039] Preparation of organic small molecule materials based on 2,6-dimethyl-4-benzonitrile acceptor unit with structural formula 2:
[0040] Compared with Example 1, the difference is that phenoxazine is replaced by an equivalent amount of 9,9-dimethyl-9,10-dihydroacridine, and other raw materials and steps are the same as in Example 1.
[0041] (1) Molecular formula of intermediate product 1: C 21 h 19 N; molecular weight: 285.39; elemental analysis results: C, 88.38; H, 6.71; N, 4.91. The reaction formula is as follows:
[0042]
[0043] (2) Molecular formula of intermediate product 2: C 21 h 18 BrN; molecular weight: 364.29; elemental analysis results: C, 69.24; H, 4.98; Br, 21.93; N, 3.85. The reaction formula is as follows:
[0044]
[0045] (3) Molecular formula of intermediate product 3: C 27 h 30 BNO 2 ; Molecular weight: 411.35; Elemental analysis results: C, 78.84; H, 7.35; B, 2.63; N, 3.41; The reaction formula is as follows:
[0046]
[0047] (...
Embodiment 3
[0050] Preparation of organic small molecule materials based on 2,6-dimethyl-4-benzonitrile acceptor unit with structural formula 3:
[0051] Compared with Example 1, the difference is that the phenoxazine is replaced by an equivalent amount of 10,10-dimethyl-5,10-dihydrodibenzo[b,e][1,4]aniline, Other raw materials and steps are all with embodiment 1.
[0052] (1) Molecular formula of intermediate product 1: C 20 h 19 NSi; molecular weight: 301.46; elemental analysis results: C, 79.68; H, 6.35; N, 4.65; Si, 9.32. The reaction formula is as follows:
[0053]
[0054] (2) Molecular formula of intermediate product 2: C 20 h 18 BrNSi; molecular weight: 380.36; elemental analysis results: C, 63.16; H, 4.77; Br, 21.01; N, 3.68; Si, 7.38. The reaction formula is as follows:
[0055]
[0056] (3) Molecular formula of intermediate product 3: C 26 h 30 BNO 2 Si; Molecular weight: 427.21; Elemental analysis results: C, 73.06; H, 7.07; B, 2.53; N, 3.28; O, 7.49; Si, 6.57....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com