Recombinant human TSG-6 protein, preparation method thereof and application of recombinant human TSG-6 protein in treatment of acute inflammatory diseases
A technology of TSG-6 and protein, applied in the biological field, can solve the problems of severe degree and harmfulness to the body, and achieve good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 TSG-6 gene cloning, expression
[0033] 1. Inoculate human embryonic lung fibroblasts (MRC-5 cells) in a 10cm cell culture dish, inoculate human cytomegalovirus (HCMV) with Moi=1, and extract total RNA on the third day after inoculating the virus;
[0034] 2. Use 500ng RNA as a template to synthesize cDNA by reverse transcription, and use self-designed specific primers to PCR amplify the full-length coding sequence of TSG-6. The specific primer sequences used are as follows:
[0035] Upstream primer: CATATGATCATCTTAATTTACTTATTTCTCTTGCTATGG
[0036] Downstream primer: CTCGAGTAAGTGGCTAAATCTTCCAGCT
[0037] 3. Connect the amplified TSG-6 coding gene to the prokaryotic expression vector pET30a to construct the recombinant expression vector pET30a-TSG-6. After sequencing and identification, transform Escherichia coli BL21(DE3) to obtain the recombinant expression strain pET30a-TSG-6- BL21;
[0038] 4. The overnight cultured recombinant bacteria were inoculate...
Embodiment 2
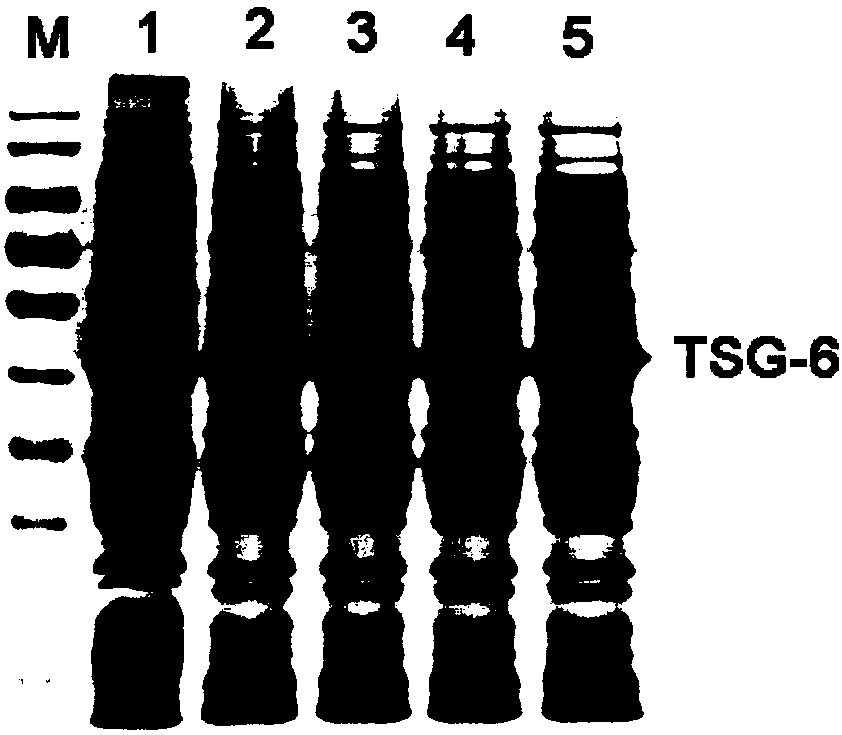
[0042] Purification of embodiment two recombinant proteins
[0043] 1. Centrifuge the enlarged cultured TSG-6 recombinant expression bacteria at 12000r / min for 10min, collect the bacteria, resuspend in PBS solution, and sonicate;
[0044] 2. Centrifuge the completely broken bacterial liquid at 12000r / min for 15min to collect the precipitate;
[0045] 3. Washing: Wash the inclusion body twice with Buffer A (50mM Tris, 100mM NaCl, 2mM urea, 0.1% EDTA, pH8.0), 3h each time; centrifuge at 12000r / min for 15min;
[0046] Denaturation: The precipitate after centrifugation was dissolved in Buffer B (50mM Tris, 100mMNaCl, 8M urea, 1% β-mercaptoethanol, pH8.0) at a ratio of 1g:20mL. After it was completely dissolved, centrifuge at 12000r / min for 15min. Discard the precipitate to obtain protein denaturation solution;
[0047]Renaturation: Dilute the resulting denaturing solution with Buffer C (50mM Tris, 100mM NaCl, 1% β-mercaptoethanol, pH8.0) to a volume ratio of 1:2, and then dilute...
Embodiment 3
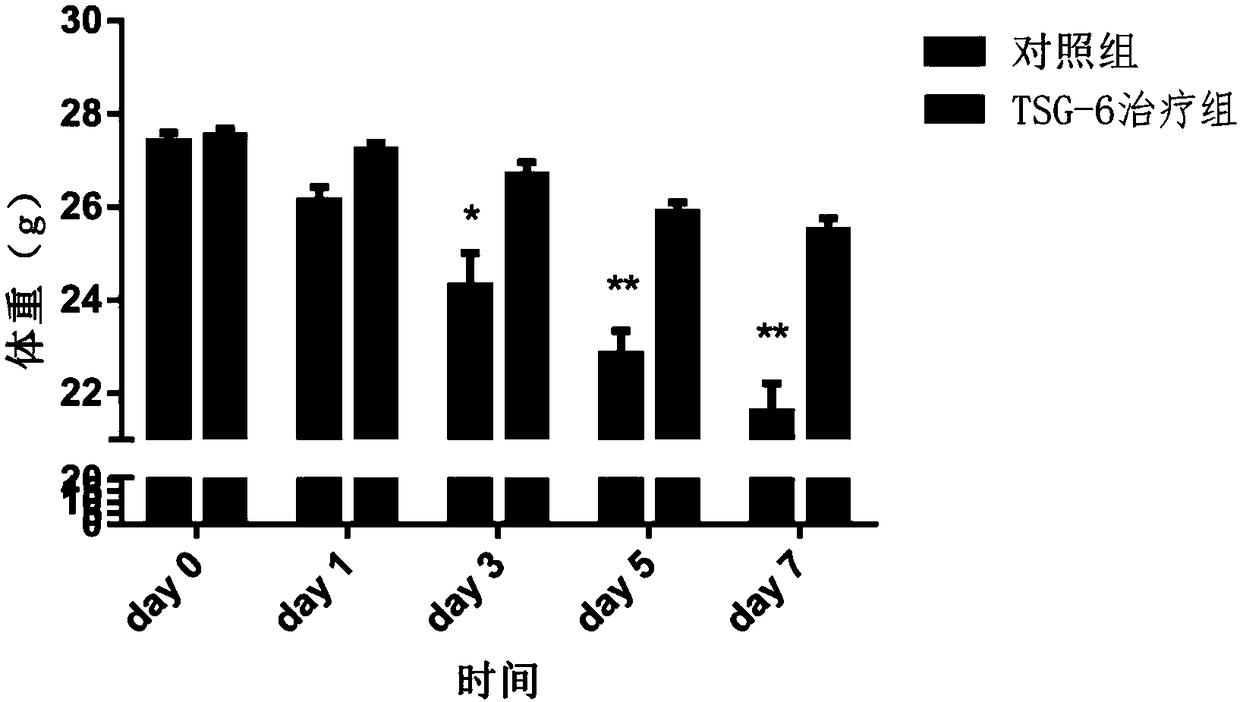
[0051] Example 3 TSG-6 recombinant protein is used to treat acute pneumonia in mice caused by HCMV infection
[0052] 1. Proliferation of HCMV
[0053] Human embryonic lung fibroblast MRC-5 1×10 5 / mL inoculated in 10cm cell culture dishes, 10mL per dish. After the cells adhered to the wall, HCMV virus was inoculated according to Moi=0.02. After incubation at 37°C for 2 h, the MEM medium containing 10% fetal bovine serum was replaced. On the third day after the virus inoculation, the MEM medium containing 3% fetal bovine serum was replaced and the culture was continued for 5-7 days. The virus-containing supernatant was harvested when the cytopathic effect of the virus reached "++++". After centrifugation at 2000r / min for 20min, the virus-containing supernatant was collected, and the number of infectious virus particles was determined by plaque formation assay.
[0054] 2. Establishment of acute pneumonia model in mice infected with HCMV
[0055] BALB / c mice of 6 to 8 wee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com