Local dense quaternized polyfluorene ether ketone compound and preparation method thereof
A ketone compound and polyfluorene ether technology, which is applied in the field of local intensive quaternization of polyfluorene ether ketone compounds and their preparation, can solve the problem that the chloromethylation reagent is highly toxic and cannot precisely control the position where the quaternary ammonium salt cation is connected. and quantity, etc., to achieve the effect of low price, excellent solubility, and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Preparation of compound I containing eight benzyl groups
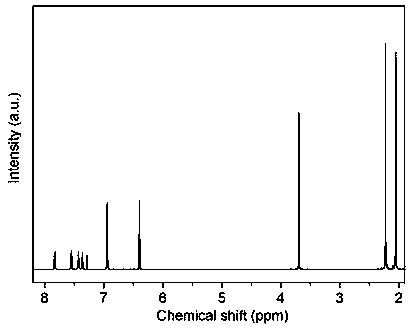
[0045]25.00 g (61.5 mmol) 9,9-bis(3,5-dimethyl-4-hydroxyphenyl) fluorene, 33.84 g (157.3 mmol) 4-bromo-2,6-dimethylanisole, Add 34.04 g (246.5 mmol) of anhydrous potassium carbonate, 5.83 g (40.6 mmol) of cuprous bromide, 21 mL (270.4 mmol) of pyridine, and 300 mL of N,N-dimethylformamide into a 500 mL three-necked flask, Under the protection of argon, stir magnetically at 165°C for 170 hours, then acidify the product to pH 2, filter, extract with water and dichloromethane, and finally purify by chromatography to obtain eight benzyl compounds I. Yield: 29.1%. The data of the proton nuclear magnetic resonance spectrum of this compound are: 1 H NMR (400 MHz, CDCl 3 , ppm)δ 2.05 (s, 12H),2.22 (s, 12H), 3.69 (s, 6H), 6.40 (s, 4H), 6.94 (s, 4H), 7.36 (t, 2H), 7.43(t, 2H), 7.55 (d, 2H), 7.83 (d, 2H).
Embodiment 2
[0046] Example 2 Preparation of bisphenol monomer II containing eight benzyl groups
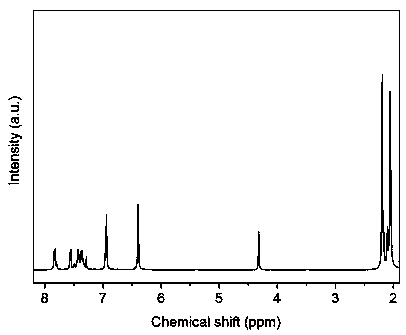
[0047] Add 5.17 g (7.66 mmol) of compound I containing eight benzyl groups in Example 1, and 130 mL of dichloromethane into a 250 mL three-necked round-bottomed flask, and use liquid nitrogen and acetone to bring the temperature of the system under the protection of argon. The temperature was lowered to -78°C, and 4.66 g (18.5 mmol) of boron tribromide was slowly added, and then the reaction was continued at room temperature for 10 hours. After the reaction, the product was poured into 200 mL of deionized water, extracted with water and methylene chloride, and purified by chromatography to obtain bisphenol monomer II containing eight benzyl groups. Yield: 99%. The data of the proton nuclear magnetic resonance spectrum of this compound are: 1 H NMR (400 MHz, CDCl 3 ,ppm) δ 2.05 (s, 12H), 2.19 (s, 12H), 4.32 (s, 2H), 6.39 (s, 4H), 6.93 (s,4H), 7.36 (t, 2H), 7.43 (t, 2H), 7.56 (d, 2H), 7.83 ...
Embodiment 3
[0048] Example 3 Preparation of polyfluorene ether ketone compound A containing benzyl
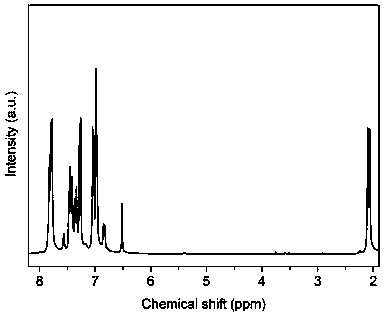
[0049] The bisphenol monomer II containing eight benzyl groups in Example 2 0.3881 g (0.6 mmol), 0.8410 g (2.4 mmol) 9,9-bis (4-hydroxyphenyl) fluorene, 0.6546 g (3 mmol) Add 4,4'-difluorobenzophenone, 0.6219 g (4.5 mmol) of anhydrous potassium carbonate, 6 mL of toluene and 10 mL of N,N-dimethylacetamide into a 25 mL three-necked flask under argon atmosphere Under protection, the temperature was raised to 145°C for 2 hours, and then the temperature was raised to 165°C to continue the reaction for 15 hours. After the reaction, the product was poured into deionized water to precipitate a precipitate, and the precipitate was collected by filtration and redissolved in dichloromethane, and then poured The precipitate was precipitated in methanol, and the precipitate was collected by filtration and dried in a vacuum oven at 80°C for 24 hours to obtain the polyfluorene ether ketone compound A co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com