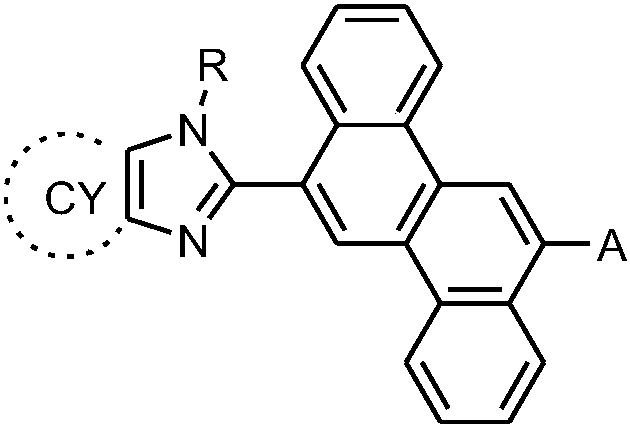
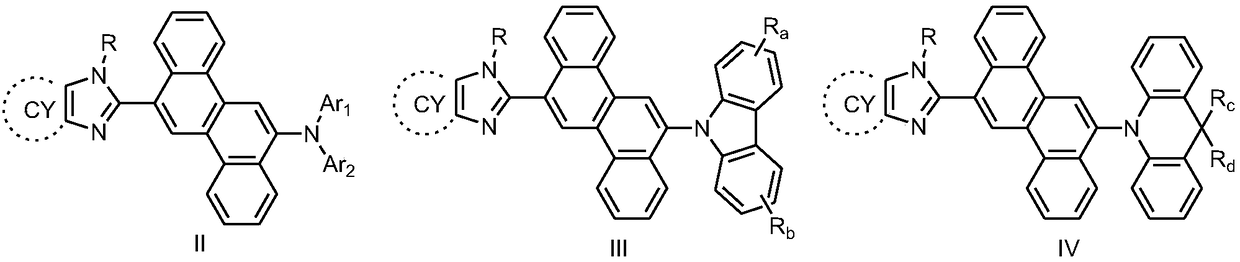
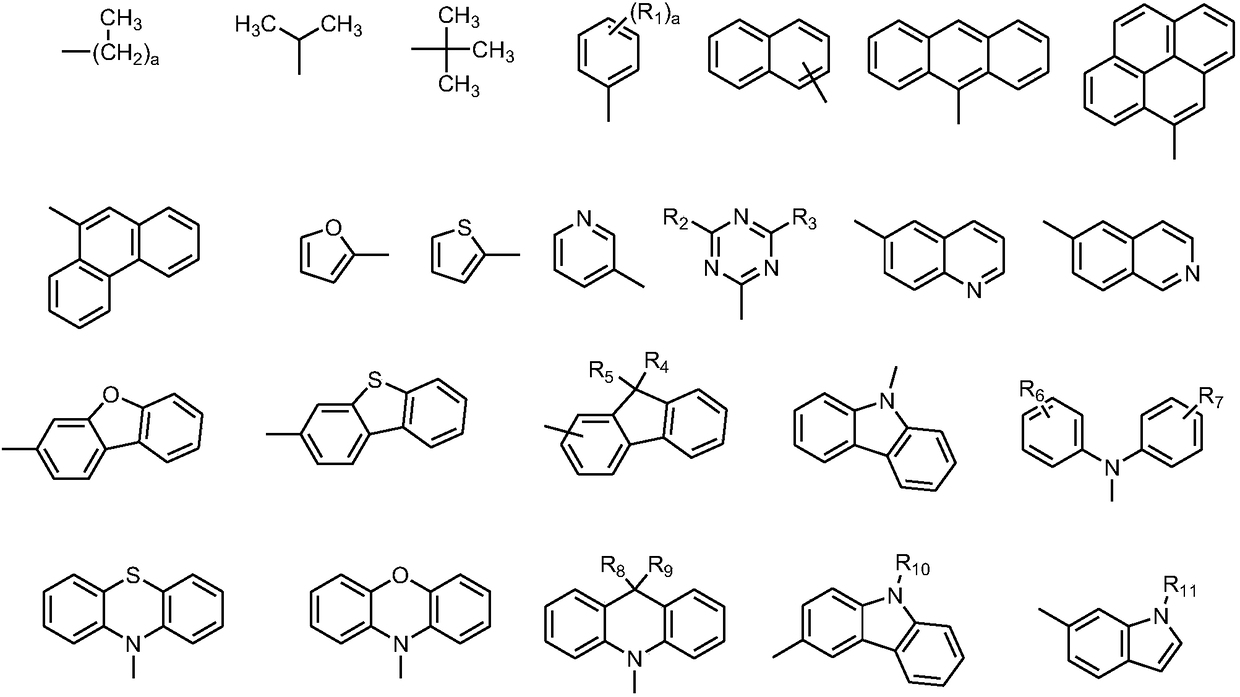
Chrysene compound containing imidazole structure and organic light-emitting device thereof
An organic light-emitting device, imidazole-containing technology, applied in the direction of light-emitting materials, organic chemistry, electric solid devices, etc., can solve the problem that the electronic energy level and the device motor energy level are poorly matched, and it is difficult to take into account the efficiency, stability and color purity, impact. Effective carrier injection and other issues, to achieve the effect of enhancing hole transport capacity, increasing electron transport capacity, and good application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] [Example 1] Synthesis of Compound A2
[0058]
[0059] Synthesis of compound a
[0060] Add 100mL THF to a three-neck flask containing 6,12-dibromochrysene (16.1g, 42.4mmol), under nitrogen protection, stir at -78°C for 30 minutes, then add 21mL n-butyllithium (2.5M) and react for 1 hour , then added 14 g of triisopropyl borate, reacted at low temperature for 1 hour, and gradually returned to room temperature. In the post-treatment process, 2M hydrochloric acid was added to the system to make the pH value of the solution 4-5, and the liquid was separated after standing, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, and spin-dried to obtain compound a (11.7g, yield 80%) .
[0061] Synthesis of compound b
[0062] Tri-tert-butylphosphine (4.4 mL of a 1.0M solution in toluene, 1.48 g, 0.05 mmol), palladium acetate (0.4 g, 1.83 mmol) and sodium tert-butoxide (22.8 g, 238 mmol) were added to 2-bromo-1H - A solution of benzimidaz...
Embodiment 2
[0070] [Example 2] Synthesis of Compound A15
[0071] The bromobenzene in Example 1 was replaced by equimolar 2-bromo-9,9-dimethylfluorene, and the other steps were the same as in Example 1 to obtain the target compound A15. Mass Spectrum m / z: 703.23 (calculated: 703.32). Theoretical element content (%)C 52 h 37 N 3 : C, 88.73; H, 5.30; N, 5.97 The measured element content (%): C, 88.72; H, 5.32; N, 5.96. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0072] [Example 3] Synthesis of Compound A24
[0073] The diphenylamine in Example 1 was replaced by equimolar 9H-carbazole, and the other steps were the same as in Example 1 to obtain the target compound A24. Mass Spectrum m / z: 585.27 (calculated: 585.22). Theoretical element content (%)C 43 h 27 N 3 : C, 88.18; H, 4.65; N, 7.17 Measured element content (%): C, 88.19; H, 4.65; N, 7.16. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com