Ternary CoAuPd catalyst for fuel cells and preparation method and application thereof
A fuel cell and catalyst technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of slow oxygen reduction reaction kinetics, large amount of noble metal Pt, and low catalyst stability, and achieve excellent catalytic oxidation of alcohols. Effects of oxygen reduction performance, improvement of catalytic performance, and ease of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
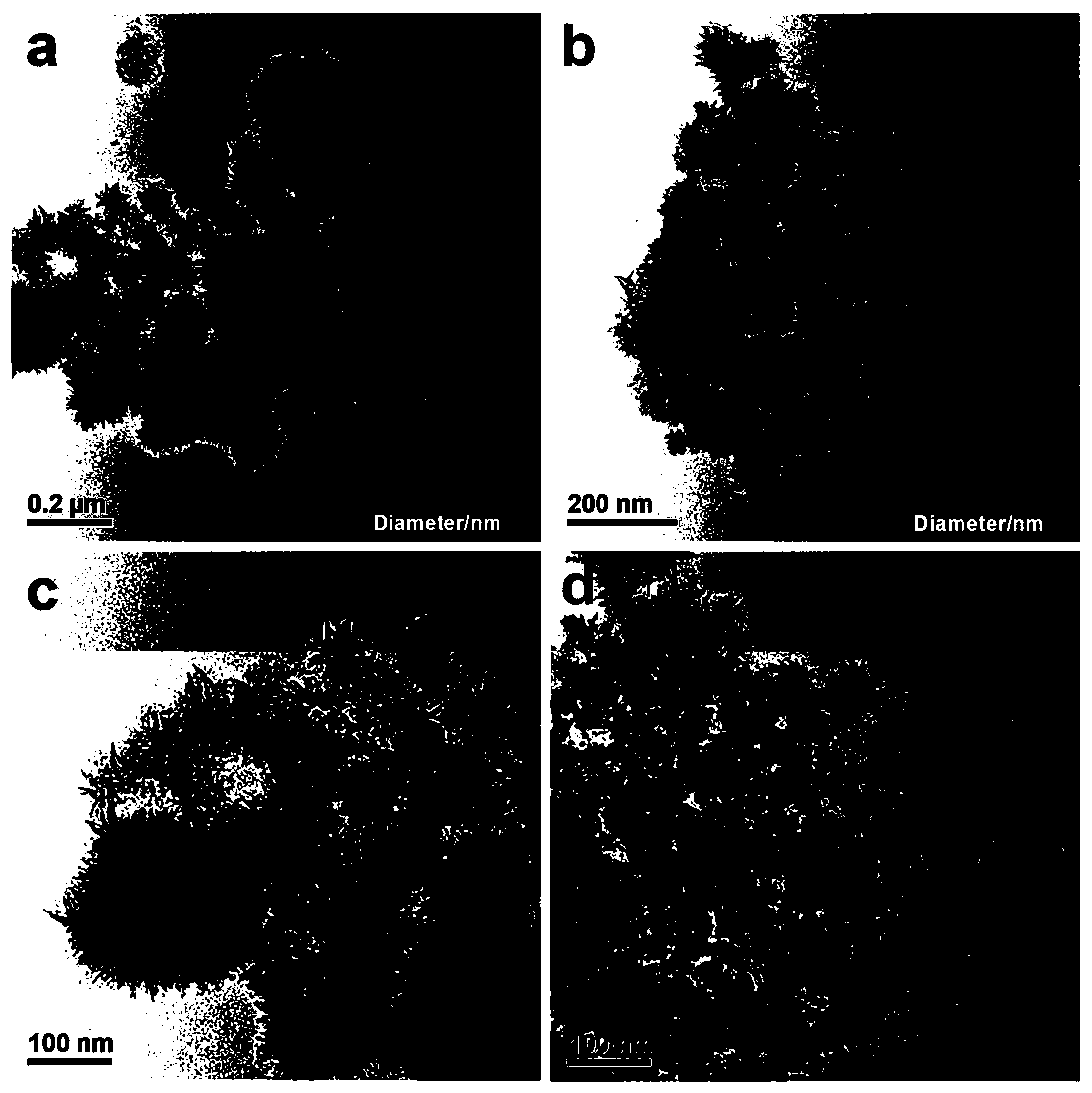
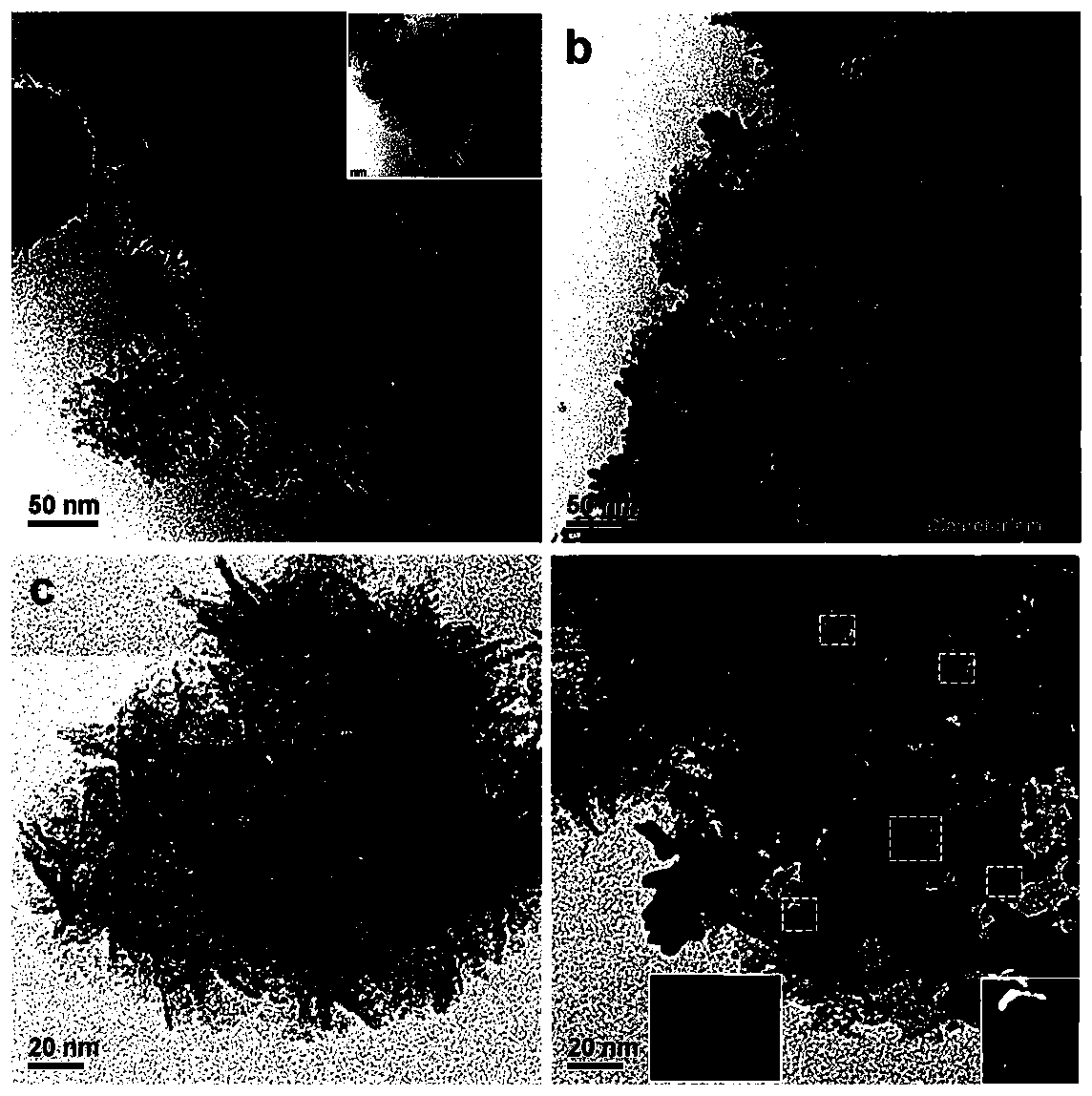
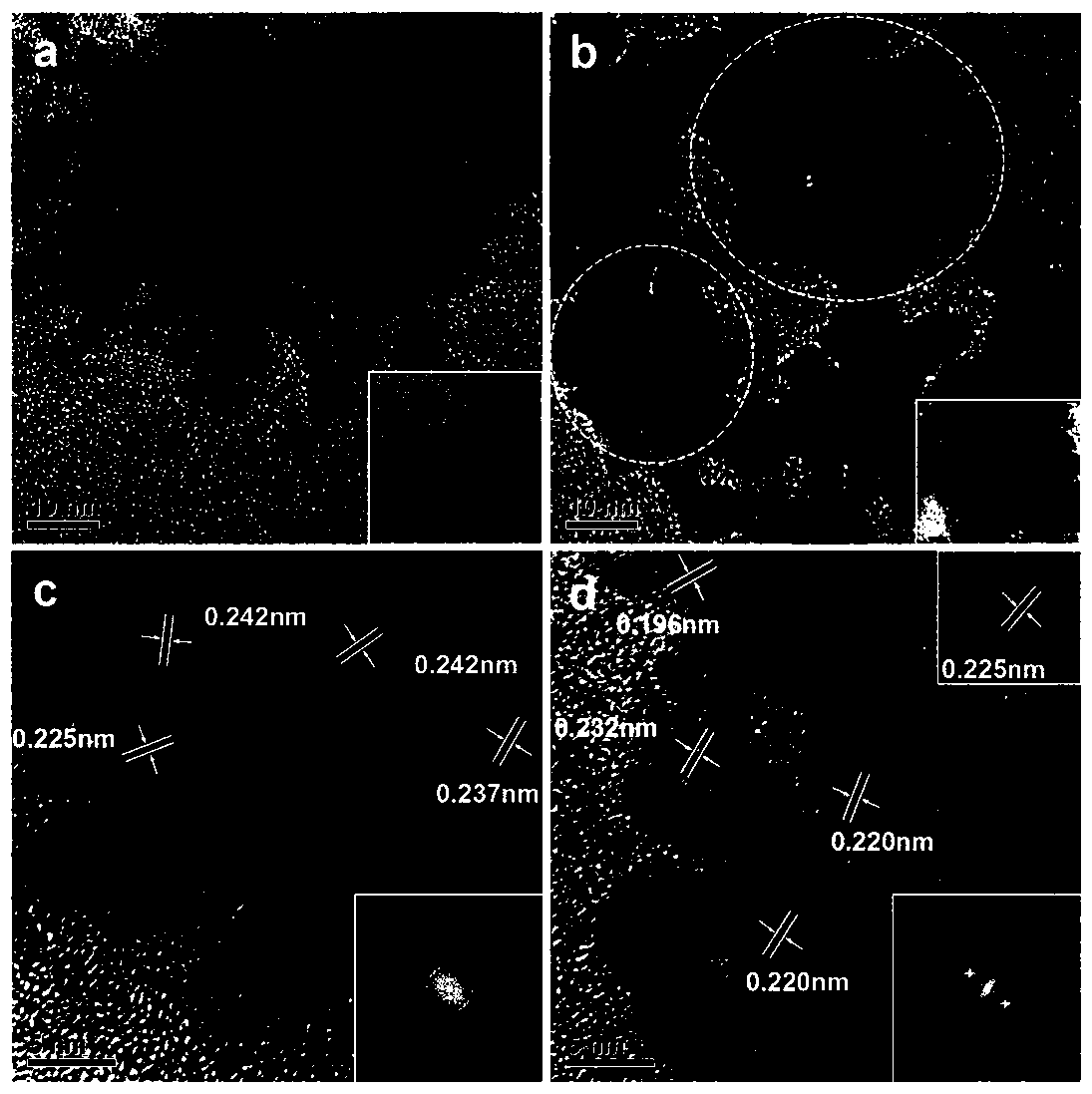
[0042] (1) Take 1.0g of triblock copolymer P123 and dissolve it in 50mL of double-distilled water with ultrasonic stirring; then add 20mL of 1.1mg / mL cobalt chloride solution, feed nitrogen to prevent Co oxidation and magnetically stir, the temperature is controlled at 30°C, Add 20 mL of sodium borohydride solution (5.0 mg / mL) dropwise at a rate of 5 s / drop into the mixed solution of P123 and cobalt chloride.
[0043] (2) After sodium borohydride has been added dropwise, after reacting for 30 minutes, add 20 mL of chloroauric acid (0.03mmol / L) and potassium chloropalladate (0.03mmol / L) dropwise at a speed of 5 s / drops in the reaction solution Mix the solution until the mixed solution turns black and a precipitate forms.
[0044] (3) After reacting for 4 hours, the obtained black suspension was centrifuged at 10000r / min, washed 3 times with double distilled water first, then 3 times with absolute ethanol, and finally the product after washing was added to anhydrous Ethanol dis...
Embodiment 2
[0053] (1) Take 1.0g of triblock copolymer P123 and dissolve it in 50mL of double-distilled water with ultrasonic stirring; then add 20mL of 1.1mg / mL cobalt chloride solution, feed nitrogen to prevent Co oxidation and magnetically stir, the temperature is controlled at 30°C, Add 20 mL of sodium borohydride solution (5.0 mg / mL) dropwise at a rate of 5 s / drop into the mixed solution of P123 and cobalt chloride.
[0054] (2) After sodium borohydride has been added dropwise, after reacting for 10 minutes, add 20 mL of chloroauric acid (0.03mmol / L) and potassium chloropalladate (0.03mmol / L) dropwise at a speed of 5 s / drops in the reaction solution Mix the solution until the mixed solution turns black and a precipitate forms.
[0055] (3) After reacting for 4 hours, the obtained black suspension was centrifuged at 10000r / min, washed 3 times with double distilled water first, then 3 times with absolute ethanol, and finally the product after washing was added to anhydrous Ethanol dis...
Embodiment 3
[0058] (1) Take 1.0g of triblock copolymer P123 and dissolve it in 50mL of double-distilled water with ultrasonic stirring; then add 20mL of 1.1mg / mL cobalt chloride solution, feed nitrogen to prevent Co oxidation and magnetically stir, the temperature is controlled at 30°C, Add 20 mL of sodium borohydride solution (10.0 mg / mL) dropwise at a rate of 5 s / drop into the mixed solution of P123 and cobalt chloride.
[0059] (2) After sodium borohydride has been added dropwise, after reacting for 50 minutes, add 20 mL of chloroauric acid (0.03mmol / L) and potassium chloropalladate (0.03mmol / L) dropwise at a speed of 5 s / drops in the reaction solution Mix the solution until the mixed solution turns black and a precipitate forms.
[0060] (3) After reacting for 4 hours, the obtained black suspension was centrifuged at 10000r / min, washed 3 times with double distilled water first, then 3 times with absolute ethanol, and finally the product after washing was added to anhydrous Ethanol di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com