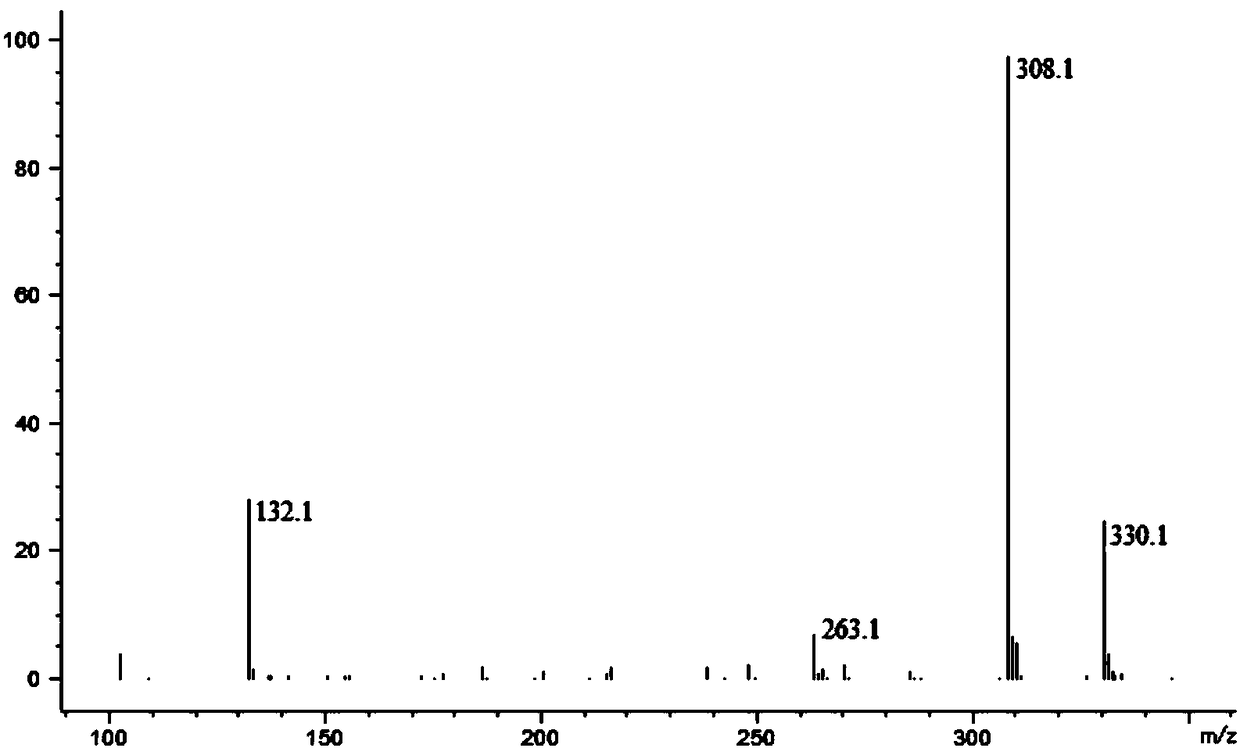
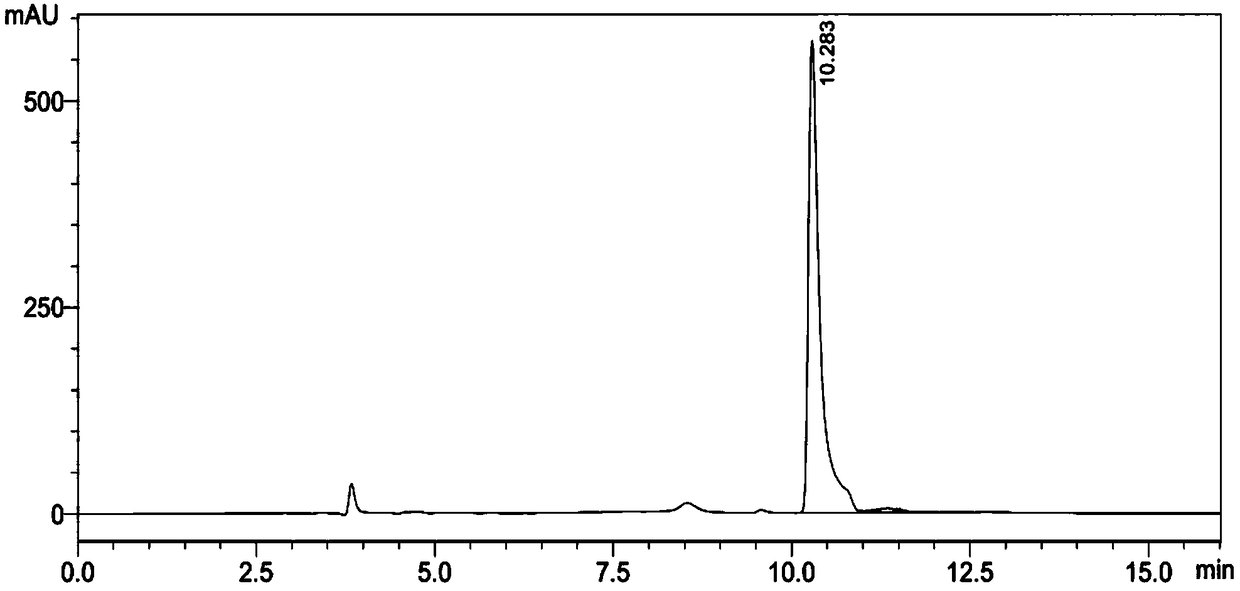
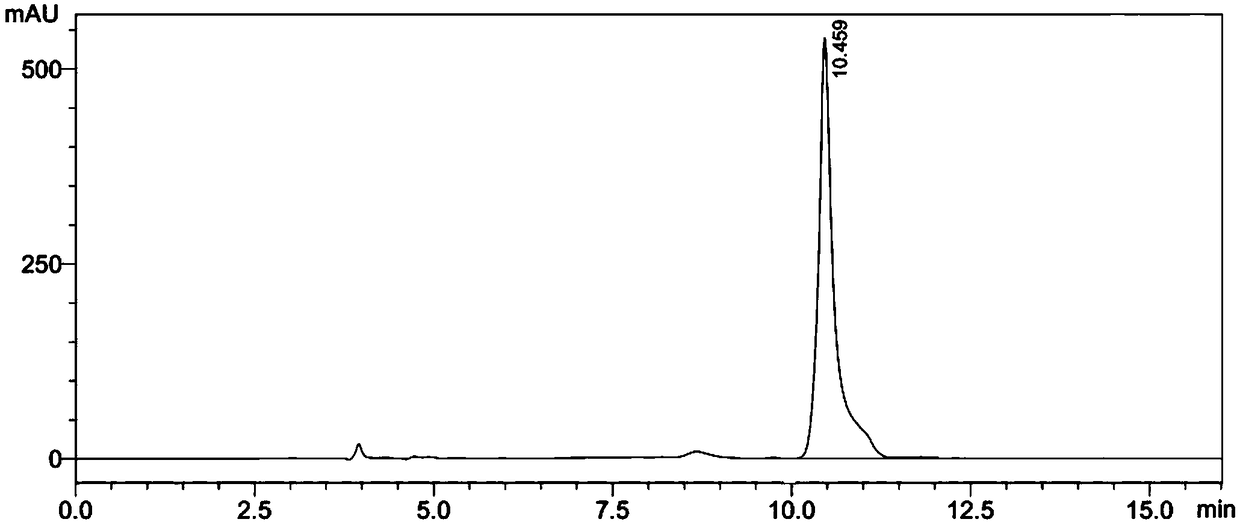
Biotransformation method for L-tert-leucine
A technology of tertiary leucine and biotransformation, applied in the field of preparation of unnatural amino acids, can solve the problems of inability to meet industrial production, no clear post-extraction process, decrease in reaction rate, etc. Feed reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation process of L-tert-leucine crystal comprises the following steps:
[0058] 1) Heat 8 parts by weight of compound glue in 50 parts by weight of distilled water to dissolve at 80°C. The compound glue includes 0.3 part by weight of calcium alginate, 0.9 part by weight of potassium alginate, 0.4 part by weight of sodium alginate and 6.4 parts by weight of poly Ethylene glycol, after cooling down to 40°C, add 1 part by weight of leucine dehydrogenase and 5.5 parts by weight of formate dehydrogenase, stir evenly, then store in the refrigerator at 4°C, let it cool and solidify, and then it will be fixed oxidized leucine dehydrogenase, formate dehydrogenase. Cut the immobilized leucine dehydrogenase and formate dehydrogenase into 1×1cm 3 cubes, spare.
[0059] 2) taking dichloropinatone as raw material to prepare sodium trimethylpyruvate solution;
[0060] Preparation of sodium trimethylpyruvate solution specifically comprises the steps:
[0061] a. Add 1 wei...
Embodiment 2
[0067] The preparation process of L-tert-leucine crystal comprises the following steps:
[0068] 1) Heat 7 parts by weight of carrageenan in 42 parts by weight of distilled water to 85°C to dissolve, add 1 part by weight of leucine dehydrogenase and 6 parts by weight of formate dehydrogenase after cooling down to 35°C, stir evenly, and place Store in the refrigerator at 4°C, let it cool and solidify, and then obtain immobilized leucine dehydrogenase and formate dehydrogenase. Cut the immobilized leucine dehydrogenase and formate dehydrogenase into 1×1cm 3 cubes, spare.
[0069] 2) Prepare sodium trimethylpyruvate solution with dichloropinatone as raw material; the preparation method is as in Example 1.
[0070] 3) Using sodium trimethylpyruvate solution as a substrate, using coenzyme NAD+ and immobilized leucine dehydrogenase and formate dehydrogenase as biocatalysts, adding ammonium formate to establish a transformation system for biotransformation reaction, and obtaining a...
Embodiment 3
[0074] The preparation process of L-tert-leucine crystal comprises the steps:
[0075] 1) Heat 9 parts by weight of compound glue in 72 parts by weight of distilled water to dissolve at 88°C. Ethylene glycol, after cooling down to 45°C, add 1 part by weight of leucine dehydrogenase and 4 parts by weight of formate dehydrogenase, stir evenly, then store in the refrigerator at 4°C, let it cool and solidify, and then it will be fixed. oxidized leucine dehydrogenase, formate dehydrogenase. Cut the immobilized leucine dehydrogenase and formate dehydrogenase into 1×1cm 3 cubes, spare.
[0076] 2) Prepare sodium trimethylpyruvate solution with dichloropinatone as raw material; the preparation method is as in Example 1.
[0077]3) Using sodium trimethylpyruvate solution as a substrate, using coenzyme NAD+ and immobilized leucine dehydrogenase and formate dehydrogenase as biocatalysts, adding ammonium formate to establish a transformation system for biotransformation reaction, and o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com