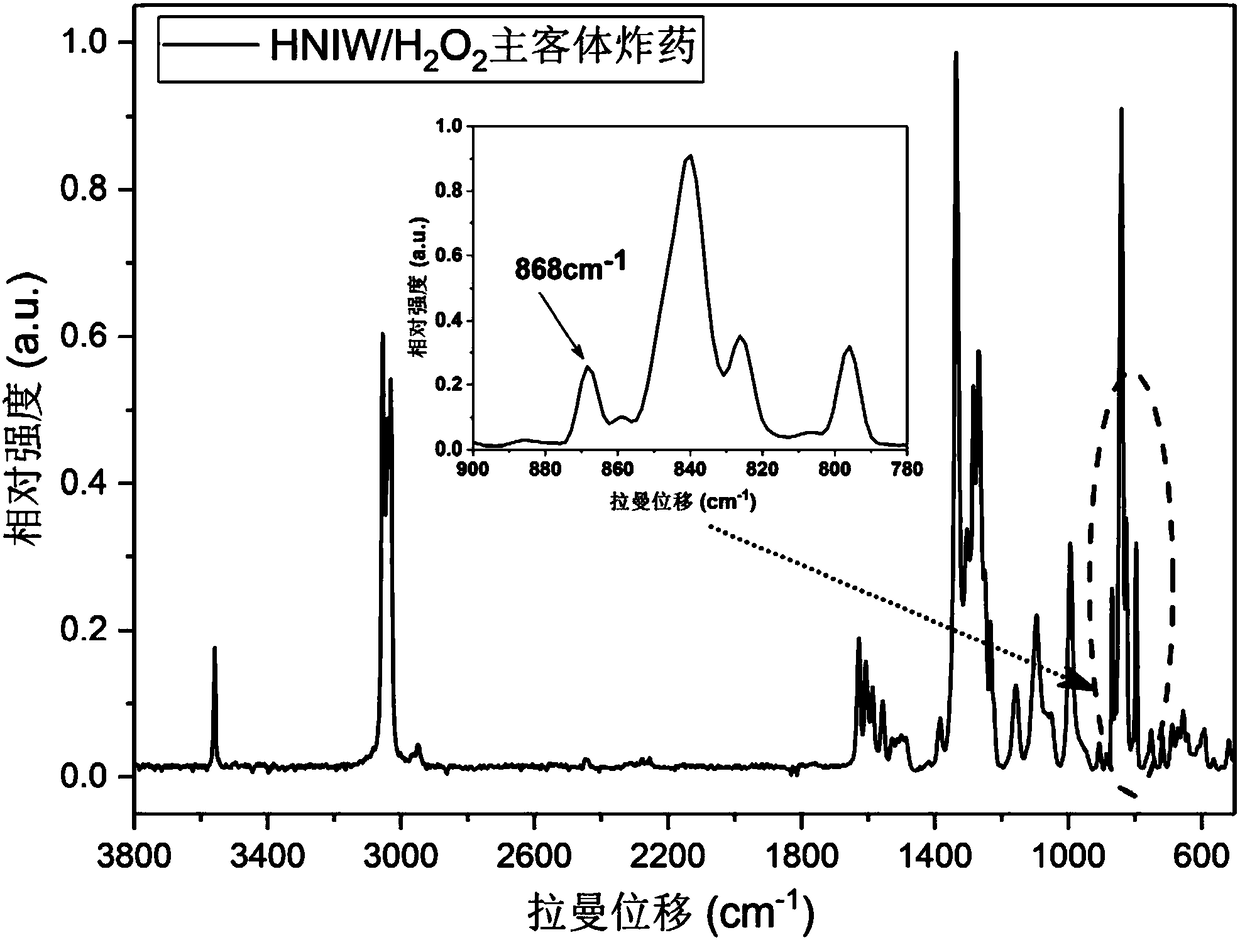
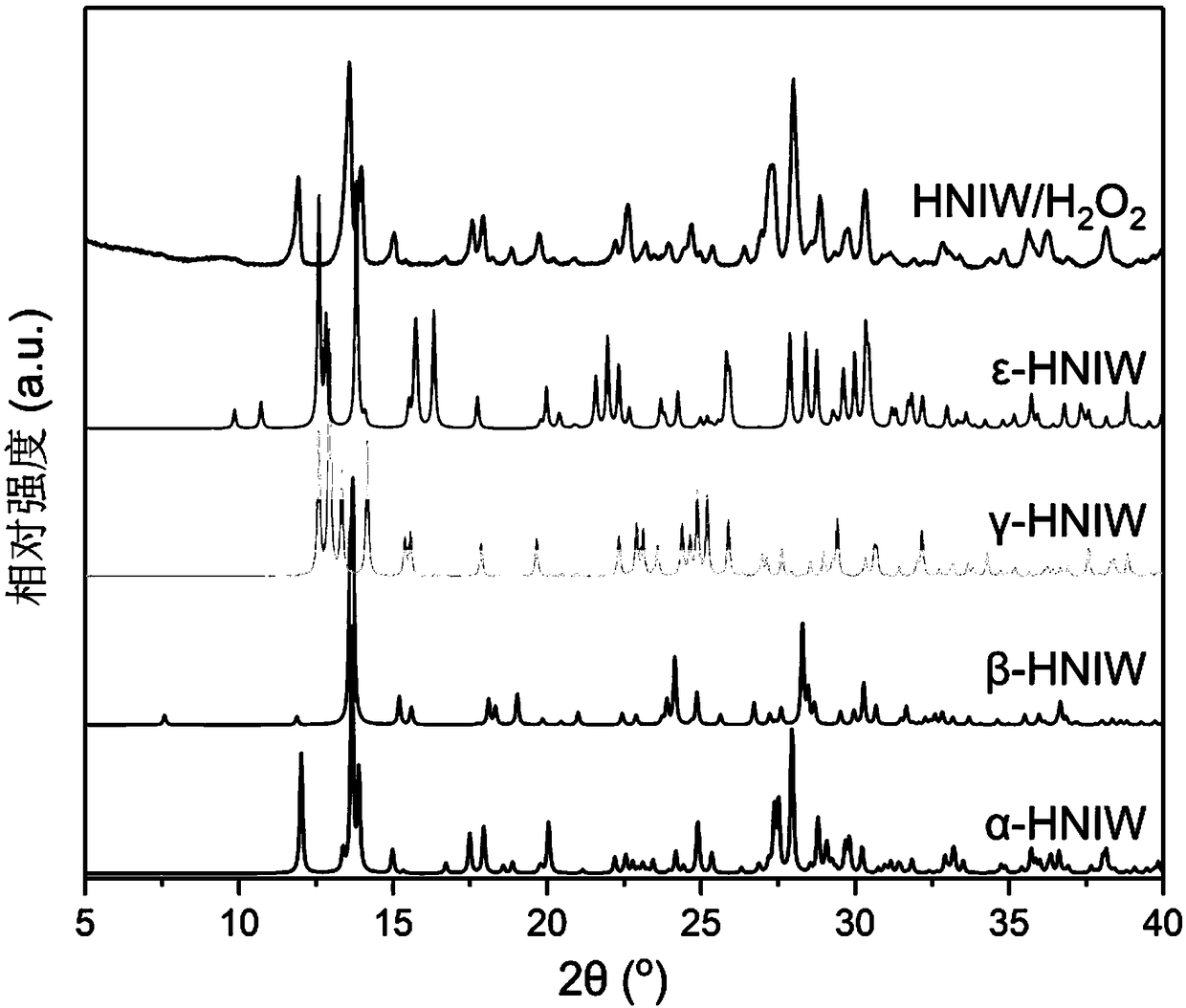
Preparation method of object and subject explosives with crystal cell embedded hydrogen peroxide molecules
A hydrogen peroxide, subject-guest technology, applied in the direction of explosives processing equipment, explosives, explosives composite components, etc., can solve the problems of hydrogen peroxide solution danger, unfavorable batch preparation, harsh experimental conditions, etc., and achieve convenient processing, molding and use , mild experimental conditions and high crystal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Weigh 10g of carbamide peroxide raw material and place it in a 150ml Erlenmeyer flask, add 60ml of anhydrous methanol and stir it with a magnetic force at 500r / min until it is completely dissolved; plug the bottle mouth tightly with a glass stopper and place it in- After standing in a constant temperature box at 10°C for 4 hours, filter and remove the precipitated urea by-product to obtain anhydrous hydrogen peroxide-containing filtrate;
[0037] (2) Weigh 5g of the HNIW raw material and place it in a 50ml Erlenmeyer flask, add 10ml of methyl acetate, and stir magnetically at 500r / min until completely dissolved to obtain an anhydrous HNIW solution;
[0038] (3) Add the HNIW solution obtained in step (2) into the hydrogen peroxide filtrate of step (1), and after fully mixing with 500r / min magnetic stirring, adopt a low-temperature evaporation crystallization method, and decompress the solution in the conical flask by a vacuum pump Distillation, the vacuum degree is m...
Embodiment 2
[0041] (1) Weigh 10g of carbamide peroxide raw material and place it in a 250ml Erlenmeyer flask, add 100ml of absolute ethanol, stir magnetically at 150r / min until completely dissolved; plug the bottle mouth tightly with a glass stopper, and place it in- After standing in a constant temperature box at 25°C for 4 hours, filter and remove the precipitated urea by-product to obtain anhydrous hydrogen peroxide-containing filtrate;
[0042] (2) Weigh 5g of HNIW raw material and place it in a 50ml Erlenmeyer flask, add 15ml of ethyl acetate, and stir magnetically at 150r / min until completely dissolved to obtain anhydrous HNIW solution;
[0043] (3) Add the hydrogen peroxide filtrate obtained in step (1) into the HNIW solution of step (2), and after fully mixing with 150r / min magnetic stirring, adopt the low-temperature evaporation crystallization method, and place the Erlenmeyer flask in a sealed desiccator , and spread 60g of color-changing silica gel on the inner bottom of the de...
Embodiment 3
[0046] (1) Take by weighing 10g of carbamide peroxide raw material and place it in a 150ml Erlenmeyer flask, measure 100ml of anhydrous diethyl ether and add, and stir magnetically at 800r / min until completely dissolved; the bottle mouth is tightly plugged with a glass stopper, and placed in a 5 After standing in a constant temperature box at ℃ for 4 hours, filter and remove the precipitated urea by-product to obtain anhydrous filtrate containing hydrogen peroxide;
[0047] (2) Weigh 5g of HNIW raw material and place it in a 50ml Erlenmeyer flask, add 20ml of ethyl formate, and stir magnetically at 800r / min until completely dissolved to obtain anhydrous HNIW solution;
[0048] (3) Add the HNIW solution obtained in step (2) into the hydrogen peroxide filtrate of step (1), and after fully mixing with 800r / min magnetic stirring, adopt a low-temperature evaporation crystallization method, and decompress the solution in the conical flask by a vacuum pump Distillation, the vacuum de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com