Coumarin derivative and preparation and application thereof
A technology of coumarin derivatives and methyl coumarin, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problem of poor control of polymer materials' hydrophilicity and hydrophobicity, and difficult control of drug coating and release To achieve the effect of good drug coating and release rate, high yield and wide application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
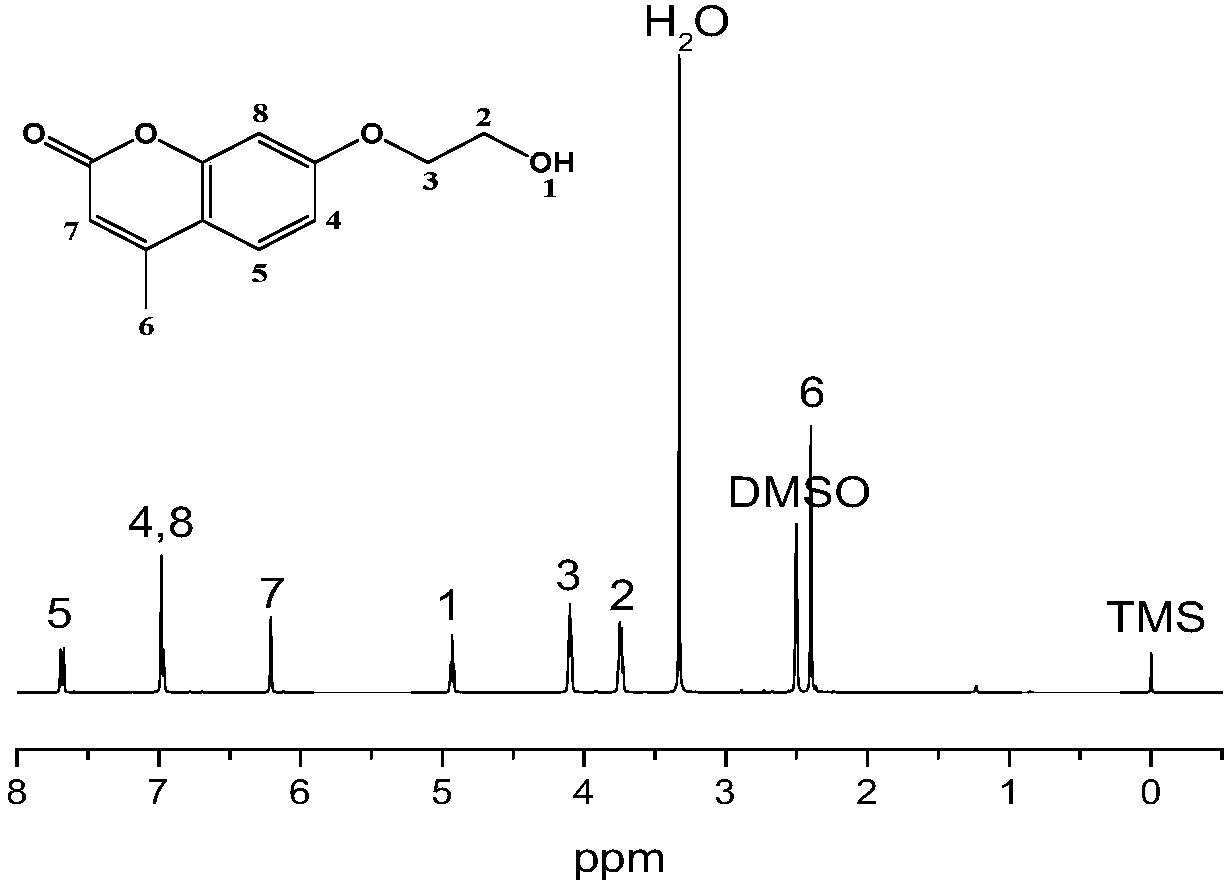
[0049] Add 7-hydroxy-4-methylcoumarin (5g, 28.38mmol), N,N-dimethylformamide (50mL) and anhydrous potassium carbonate (3g) into the reaction tube, ultrasonically disperse for 10 minutes and use Seal the tube with a rubber stopper. The reaction tube was vigorously stirred at 85°C. After the system was stabilized and mixed uniformly, 2-bromoethanol (2.84ml, 40mmol) was injected dropwise into the reaction tube with a disposable syringe, and the reaction was vigorously stirred for 20 hours. After the reaction was completed, filter, and then drop the filtrate into ice deionized water, a white solid precipitated, washed with ice deionized water several times, and vacuumed to constant weight to obtain the intermediate 7-(2-hydroxyethoxy)-4-methanol base coumarin.
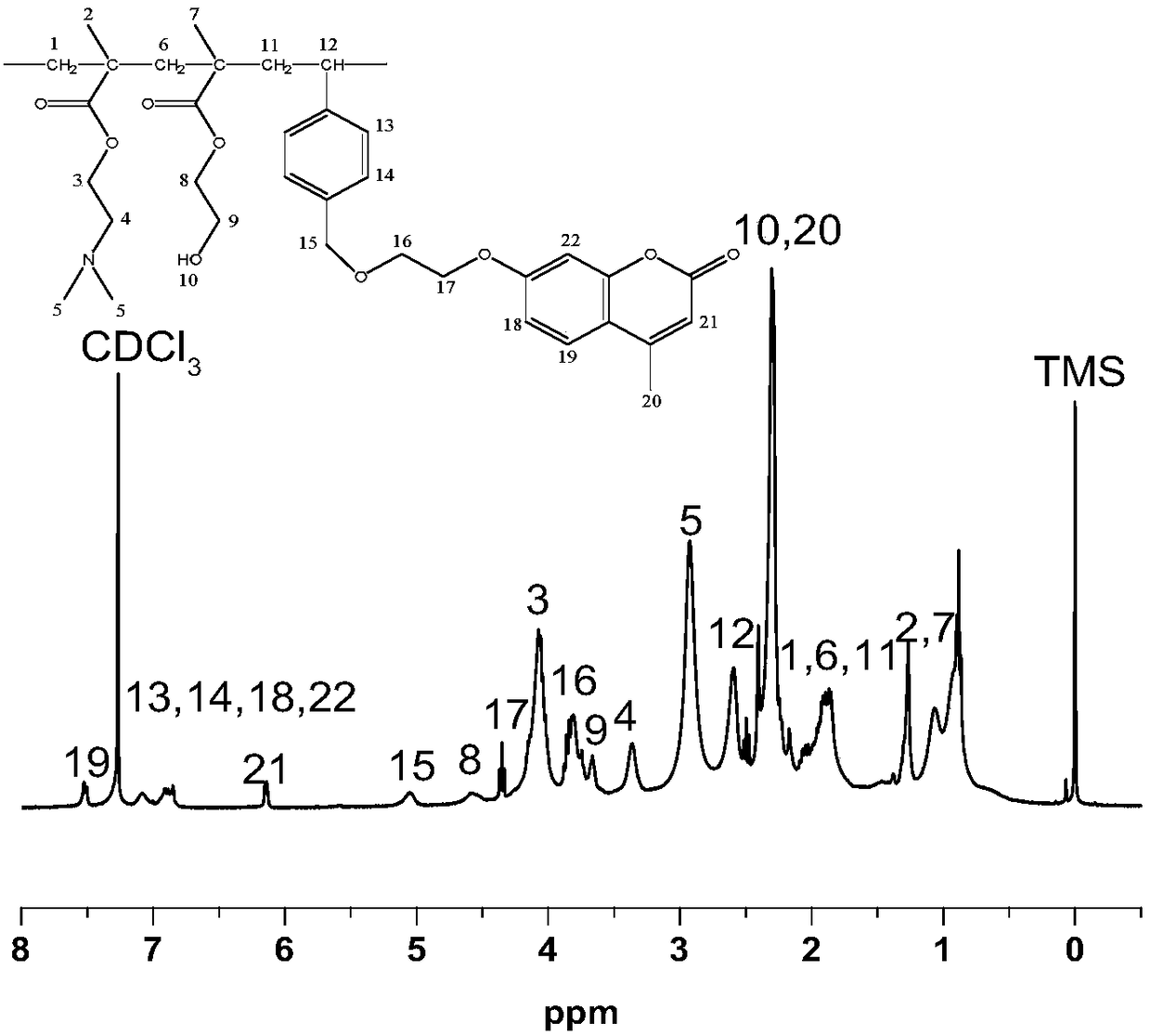
[0050] Add 7-(2-hydroxyethoxy)-4-methylcoumarin (1.1g, 5mmol), N,N-dimethylformamide (20ml), bis(2,6-bis tert-butyl-4-methylphenyl) pentaerythritol diphosphate (0.11g) and anhydrous potassium carbonate (0.83g), after ult...
Embodiment 2
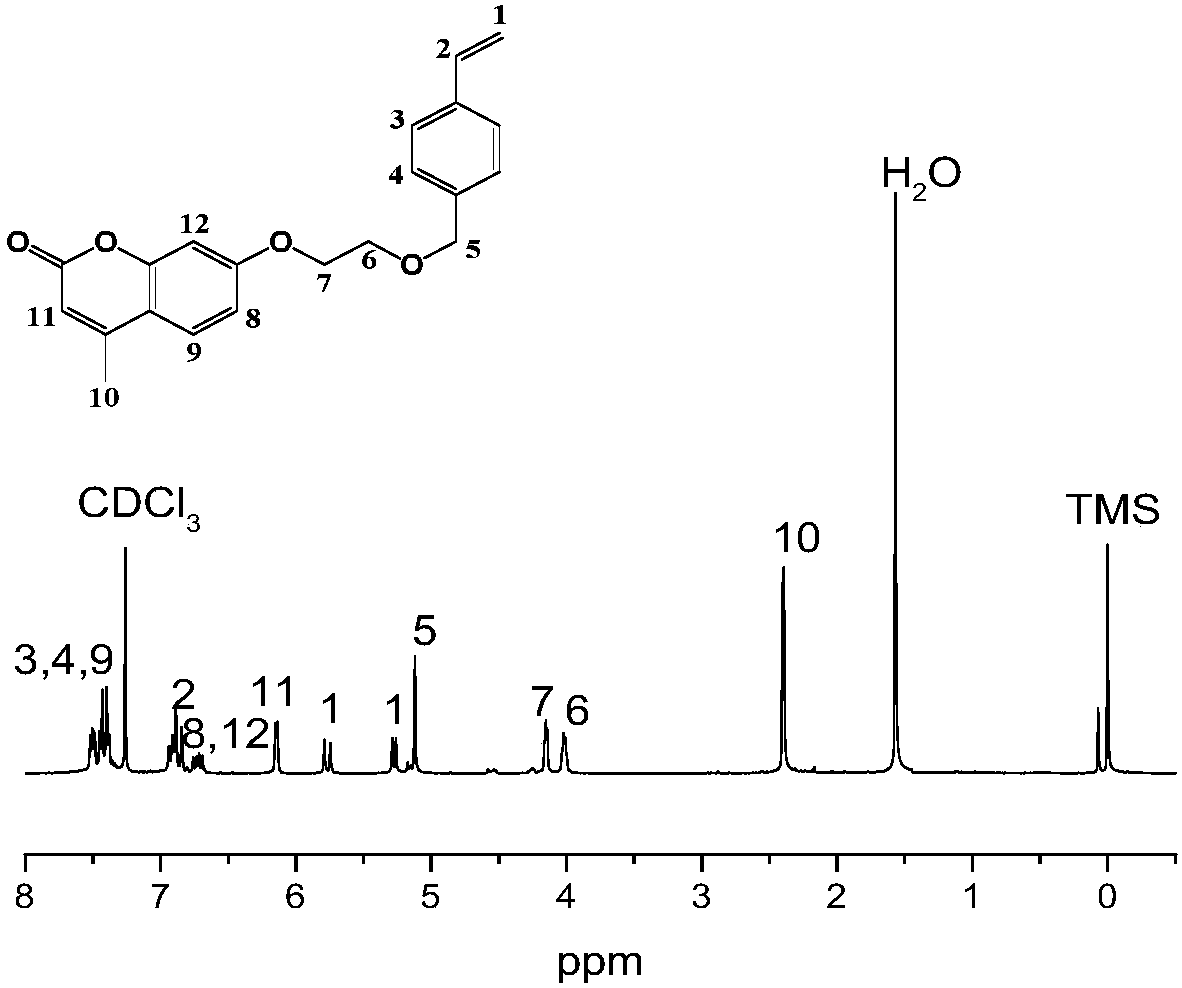
[0052] According to the first step of the substitution reaction in Example 1, the intermediate 7-(2-hydroxyethoxy)-4-methylcoumarin was obtained.
[0053] Add 7-(2-hydroxyethoxy)-4-methylcoumarin (1.1g, 5mmol), N,N-dimethylformamide (20ml), bis(2,6-bis tert-butyl-4-methylphenyl) pentaerythritol diphosphate (0.11g) and anhydrous potassium carbonate (0.83g), after ultrasonic dispersion for 10 minutes, the tube was sealed with a rubber stopper, after which the reaction tube was vigorously stirred at 65°C , Introduce high-purity nitrogen for 20 minutes to remove oxygen in the reaction system and prevent 4-vinylbenzyl chloride from exploding after being mixed with air due to its active chemical properties. After the system was stabilized and mixed uniformly, 4-vinylbenzyl chloride (1.137ml, 8mmol) was injected dropwise into the reaction tube with a disposable syringe, and stirred vigorously at 65°C for 24 hours. After the reaction is finished, filter, then drop the filtrate into i...
Embodiment 3
[0055] According to the first step of the substitution reaction in Example 1, the intermediate 7-(2-hydroxyethoxy)-4-methylcoumarin was obtained.
[0056] Add 7-(2-hydroxyethoxy)-4-methylcoumarin (1.1g, 5mmol), N,N-dimethylformamide (20ml), bis(2,6-bis tert-butyl-4-methylphenyl) pentaerythritol diphosphate (0.11g) and anhydrous potassium carbonate (0.83g), after ultrasonic dispersion for 10 minutes, the tube was sealed with a rubber stopper, after which the reaction tube was vigorously stirred at 65°C , Introduce high-purity nitrogen for 20 minutes to remove oxygen in the reaction system and prevent 4-vinylbenzyl chloride from exploding after being mixed with air due to its active chemical properties. After the system was stabilized and mixed uniformly, 4-vinylbenzyl chloride (1.42ml, 10mmol) was injected dropwise into the reaction tube with a disposable syringe, and stirred vigorously at 65°C for 24 hours. After the reaction is finished, filter, then drop the filtrate into i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com