Carbohydrate alkyl bonding imidazole type heterocyclic carbene palladium coordination compound as well as preparation method and application thereof
A carbohydrate and alkyl bond technology, applied in the field of synthesis and catalysis of chiral nitrogen heterocyclic carbene transition metal complexes, can solve the problems of increasing complexity, violating the principle of environmental protection, atom economy, reducing the total yield of the reaction, etc. problems, to achieve the effects of convenient post-treatment, good substrate universality and functional group tolerance, good catalytic activity and reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
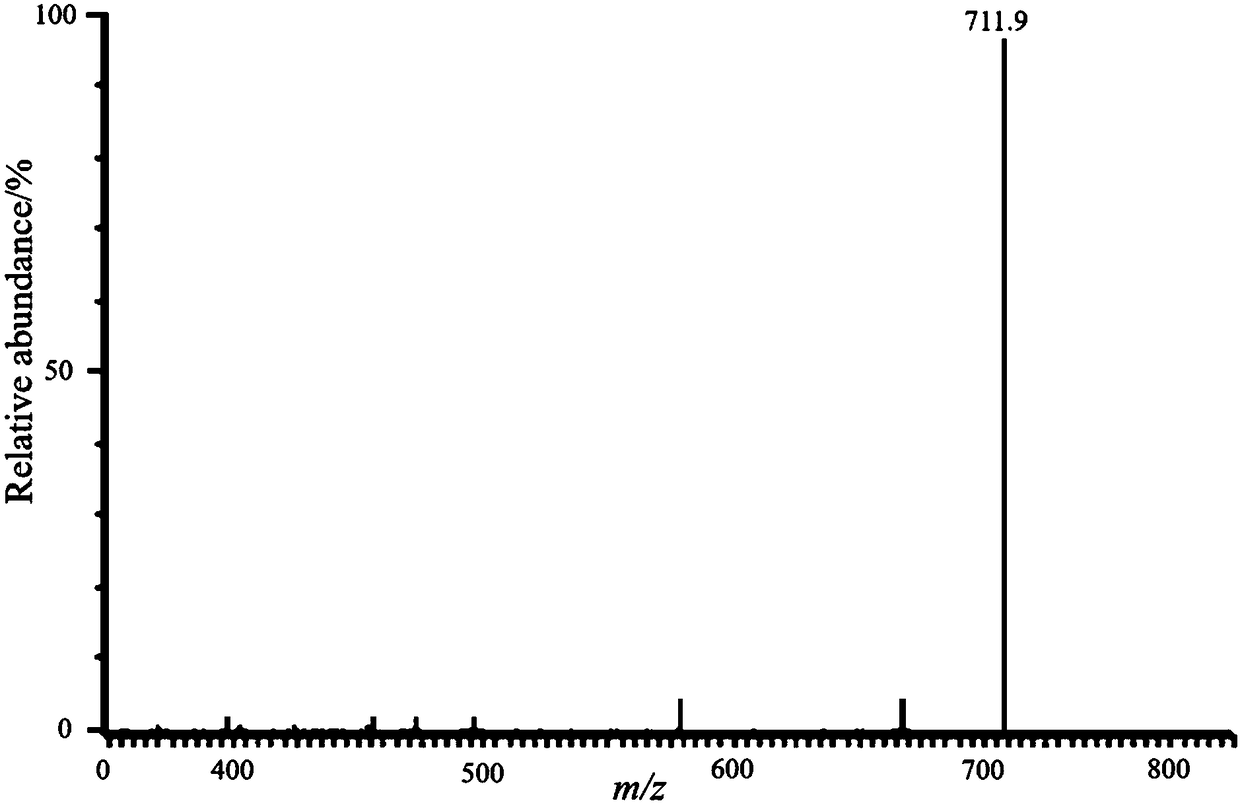
[0030] This embodiment relates to a one-step preparation method of a carbohydrate alkyl-linked imidazole-type azacyclic carbene palladium complex, which includes the following steps:
[0031] Under nitrogen protection, 1.0 mmol brominated 1-[2-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy )-ethyl]-3-methylimidazolium salt, 1.0mmol of palladium acetate, 3.0mmol of dry pyridine, vigorously reflux reaction in 10mL of dry THF for 4 hours, thin-layer chromatography tracking (methylene chloride:methanol=20:1 ), after the reaction was complete, stop the reaction, post-treatment, column chromatography purification (methylene chloride: methanol = 20: 1), to obtain yellow viscous compound I-1, yield 95%, 1 H NMR (400MHz, CDCl 3)δ1.97(s,3H),1.99(s,3H),2.00(s,3H),2.04(s,2H),2.08(s,3H),3.70(m,1H),4.08(s,3H ),4.12(m,2H), 4.25(dd,J 1 =12.0Hz,J 2 =4.8Hz, 1H), 4.33(m, 3H), 4.52(d, J=8.0Hz, 2H), 4.98(m, 2H).5.06(t, J=14.0Hz, 1H), 5.16(t, J =9.2Hz,1H),6.86(d,J=2.0Hz,1H),6.98(d,J=2.3Hz,1H),7...
Embodiment 2
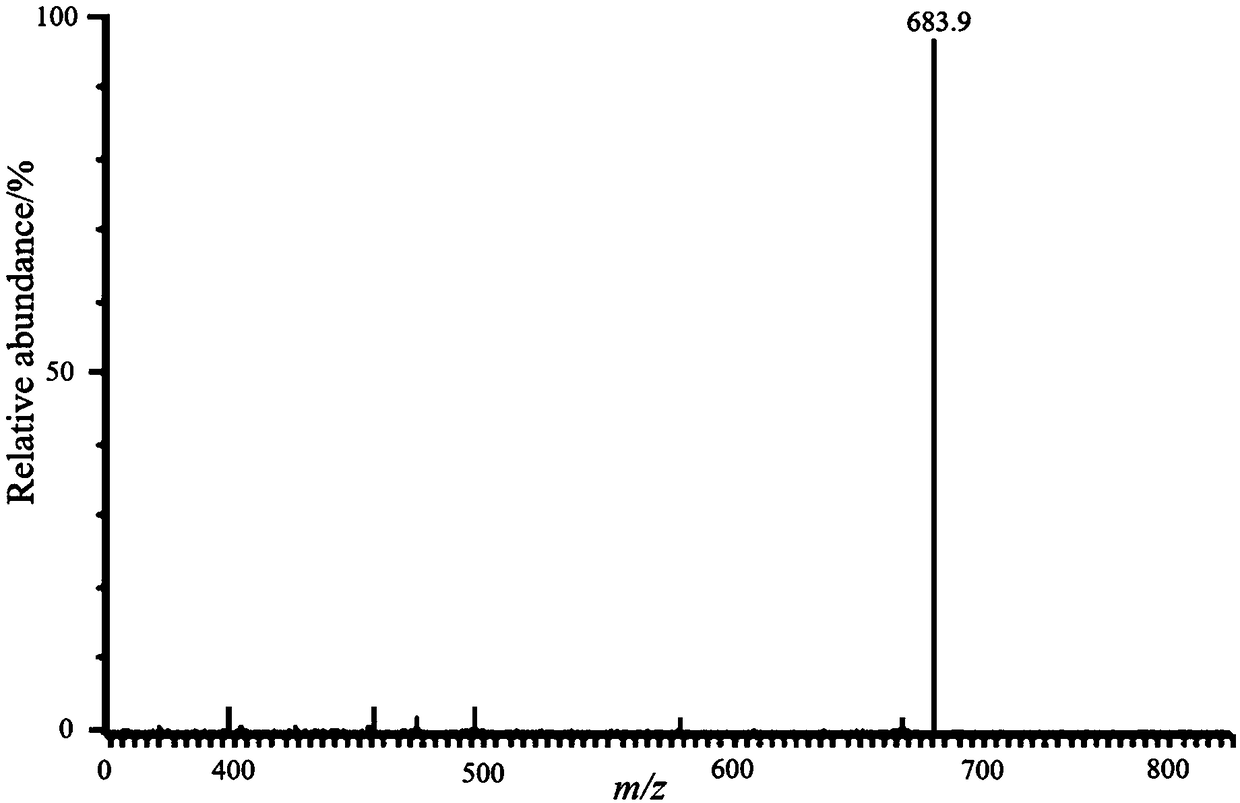
[0034] This embodiment relates to a method for preparing a carbohydrate alkyl-linked imidazole-type azacyclic carbene palladium complex, which comprises the following steps:
[0035] Add 1.0 mmol of brominated 1-[2-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-ethyl ]-3-butyl imidazolium salt, palladium acetate of 1.0mmol, dry pyridine of 3.0mmol, violent reflux reaction in 10mL dry THF 4 hours, thin-layer chromatographic tracking (dichloromethane:methanol=20:1), reaction is complete Afterwards, stop the reaction, work up, and purify by column chromatography (dichloromethane:methanol=20:1) to obtain yellow viscous compound I-2 with a yield of 93%. 1 H NMR (400MHz, CDCl 3 )δ1.01(t, J=7.6Hz, 3H), 1.23 (t, J=7.2Hz, 4H), 1.46(m, 2H), 1.94(s, 3H), 1.97(s, 3H), 1.99( s,3H),2.02(s,3H), 2.07(s,3H),3.69(m,1H),4.10(m,2H),4.23(m,1H),4.29(m,1H),4.42(m ,3H),4.52(m,2H),4.98(m,2H).5.05(t,J=10.0Hz,1H),5.15(t,J=9.2Hz,1H),6.87(d,J=6.0Hz ,1H),6.98(d,J=2.0Hz,1H),7.32(m,2H),7.75(m,1H),8.99(m,...
Embodiment 3
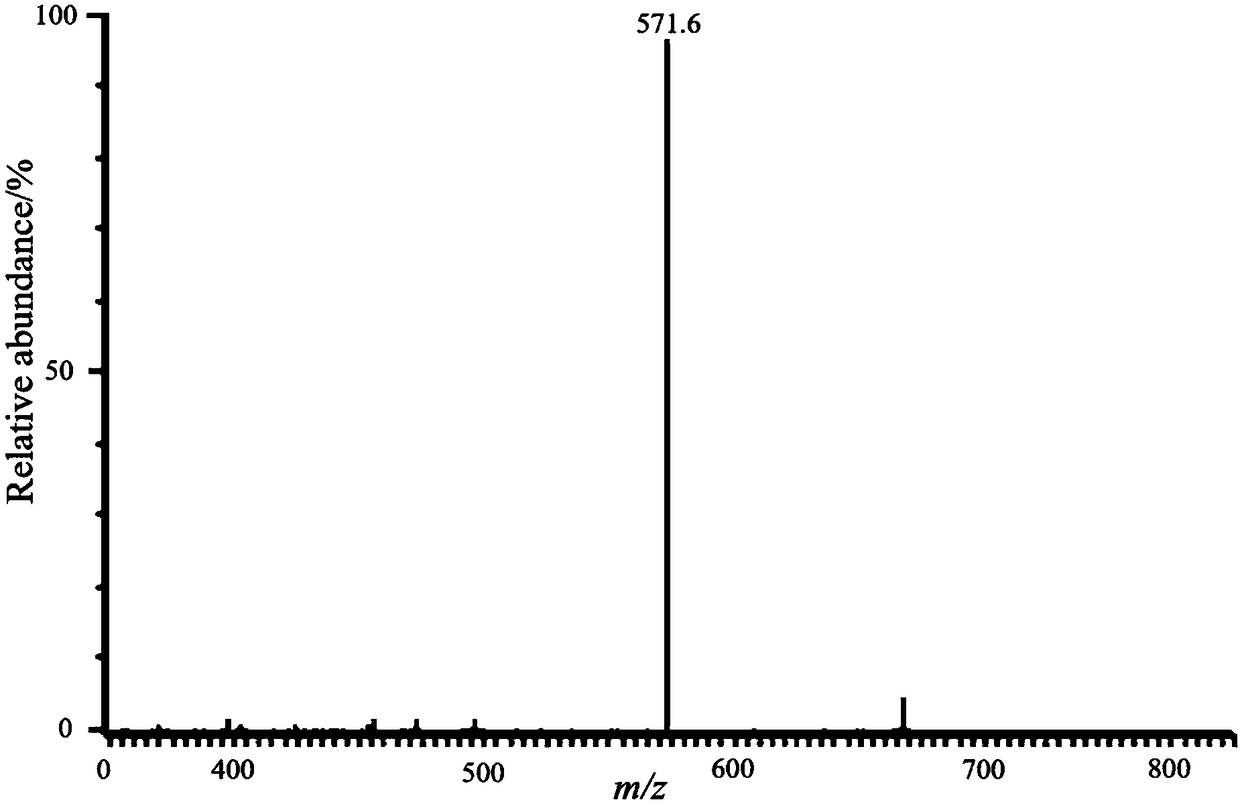
[0038] This embodiment relates to a method for preparing a carbohydrate alkyl-linked imidazole-type azacyclic carbene palladium complex, which comprises the following steps:
[0039] Add 1.0 mmol of brominated 1-[2-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-ethyl ]-3-phenylimidazolium salt, palladium acetate of 1.0mmol, dry pyridine of 3.0mmol, violent reflux reaction in 10mL dry THF 4 hours, thin-layer chromatographic tracking (dichloromethane:methanol=20:1), reaction is complete Afterwards, the reaction was stopped, after-treatment, and column chromatography purification (dichloromethane:methanol=20:1) to obtain yellow viscous compound I-3 with a yield of 91%; 1 H NMR (400MHz, CDCl 3 )δ2.00(s,3H),2.01(s,3H), 2.02(s,3H),2.04(s,3H),2.09(s,3H),3.72(m,1H),4.10(m,3H ),4.28(m,1H),4.41(m,1H),4.52(m,2H),4.59(d,J=8.0Hz,2H).5.05(t,J=10.0Hz,1H),5.12(t ,J=10.0Hz,1H),5.19(t,J=8.8Hz,1H),7.14(d,J=2.0Hz,1H),7.18(d,J=2.4Hz,1H), 7.26(m,2H ),7.48(m,1H),7.58(t,J=8.0Hz,2H),7.70(m,1H),8.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com