Perovskite oxide and preparation thereof, and application during photothermal chemical conversion of solar energy
A technology of perovskite oxides and oxides, applied in inorganic chemistry, cobalt compounds, carbon monoxide, etc., can solve the problems of less oxygen vacancies, high reduction temperature of metal oxides, and cycle stability to be improved, and achieve a synthesis method. Simple, easy to synthesize effects at scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
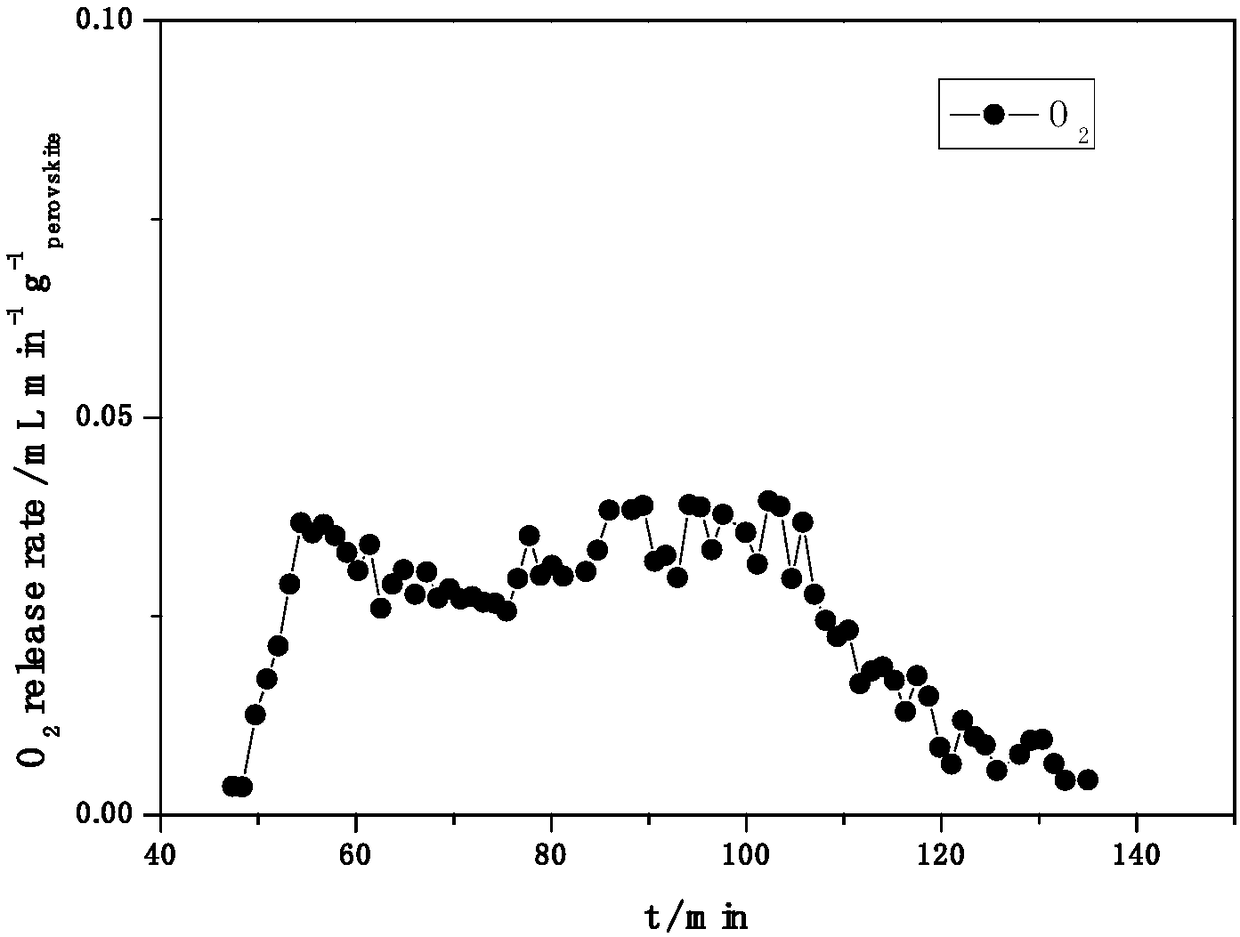
Image
Examples
Embodiment 1
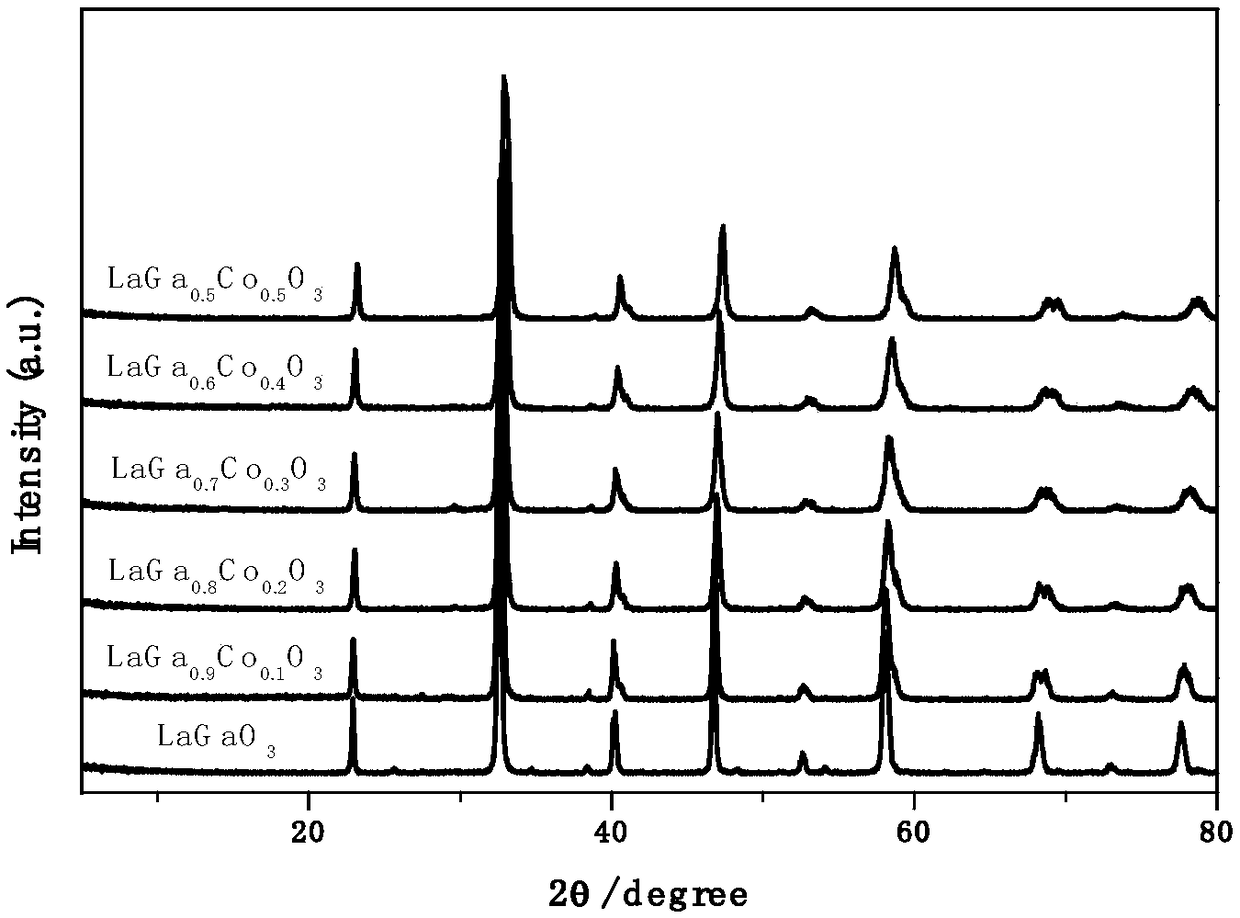
[0038] Weigh 4.3301g (10mmol) lanthanum nitrate, 0.4177 (10-n) g ((10-n) mmol) gallium nitrate, 0.2910 n g (nmmol, n=0-10) cobalt nitrate and dissolve in 20-100ml deionized water, Stir at room temperature for 30 minutes; at 60-100°C, evaporate the excess water to a sol-like substance; transfer to a muffle furnace at 500°C for ignition, and heat-preserve and roast for 0.5-2h; then, at a heating rate of 2-5°C Raise the temperature to 800°C, and heat-preserve and roast for 3-6h. Select different types of rare earth metal or alkaline earth metal nitrates (such as one or more of metal nitrates such as La, Nd, Sm, Gd, Dy, Y, Sr, Ca, Ba, Bi, Ce, etc.) and transition metals Or alkaline earth metal nitrates (such as Fe, Mn, Cu, Co, Ni, Al, Cr, Sc, Mg and other metal nitrates or two or more); adjust the doping of different elements in the A-position and B-position Impurity ratio (0-100atom%); a series of A-site and B-site doped perovskite oxide AGa 1-x B x o 3-δ , with figure 2 XR...
Embodiment 2
[0040]Weigh 4.3301g (10mmol) lanthanum nitrate, 0.4177 (10-n) g ((10-n) mmol) gallium nitrate, 0.2910 n g (nmmol, n=0-10) cobalt nitrate and dissolve in 20-100ml deionized water, Stir at room temperature for 30 minutes; then add NaOH solution or KOH solution or ammonia water with a concentration of 1-3mol / L, adjust the pH of the solution to 7.0-12.0, stir evenly; transfer to a hydrothermal kettle, and react at 80°C-200°C for 3- 12h; after cooling to room temperature, filter, wash and dry. Select different types of rare earth metal or alkaline earth metal nitrates (such as one or more of metal nitrates such as La, Nd, Sm, Gd, Dy, Y, Sr, Ca, Ba, Bi, Ce, etc.) and transition metals Or alkaline earth metal nitrates (such as Fe, Mn, Cu, Co, Ni, Al, Cr, Sc, Mg and other metal nitrates or two or more); adjust the doping of different elements in the A-position and B-position Impurity ratio (0-100atom%); a series of A-site and B-site doped perovskite oxide AGa 1-x B x o 3-δ .
Embodiment 3
[0042] Weigh 3.2581g (10mmol) lanthanum oxide, 0.1874 (10-n) g ((10-n) mmol) gallium oxide, 0.1189ng (nmmol, n=0-10) cobalt carbonate; grind evenly with a mortar, Under the protection of a certain atmosphere, heat to 1100°C at a rate of 10-20°C / min, keep it warm for 6-12h, and then cool down to room temperature; the composition of the atmosphere is: the volume ratio is air (0-100%) and CO 2 (100-0%) mixed gas, the total flow rate is 100mL / min. Select different types of rare earth metal or alkaline earth metal carbonates or oxides (such as La, Nd, Sm, Gd, Dy, Y, Sr, Ca, Ba, Bi, Ce and other metal carbonates or oxides) or two or more) and transition metal or alkaline earth metal carbonates or oxides (such as Fe, Mn, Cu, Co, Ni, Al, Cr, Sc, Mg and other metal carbonates or oxides or one or both more than one species); adjust the doping ratio (0-100atom%) of different elements of A-site and B-site; obtain a series of A-site and B-site doped perovskite oxide AGa 1-x B x o 3-δ ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com