Piperazine-modified tetraphenylethylene derivative and application thereof
A technology of tetraphenylethylene and triphenylethylene, which is applied in the field of organic photoelectric functional materials, can solve problems such as red blood cell destruction and human poisoning, and achieve the effects of rapid response, short response time, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
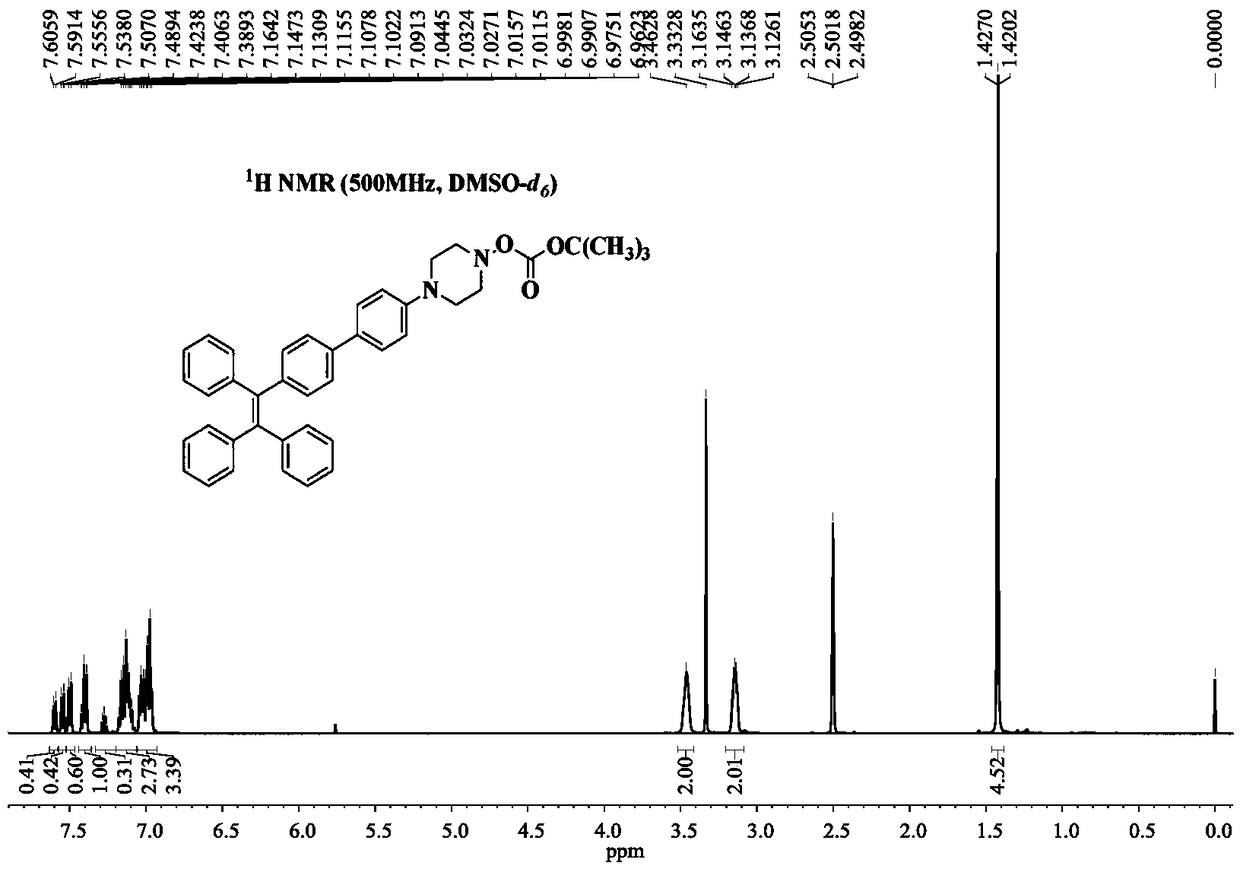
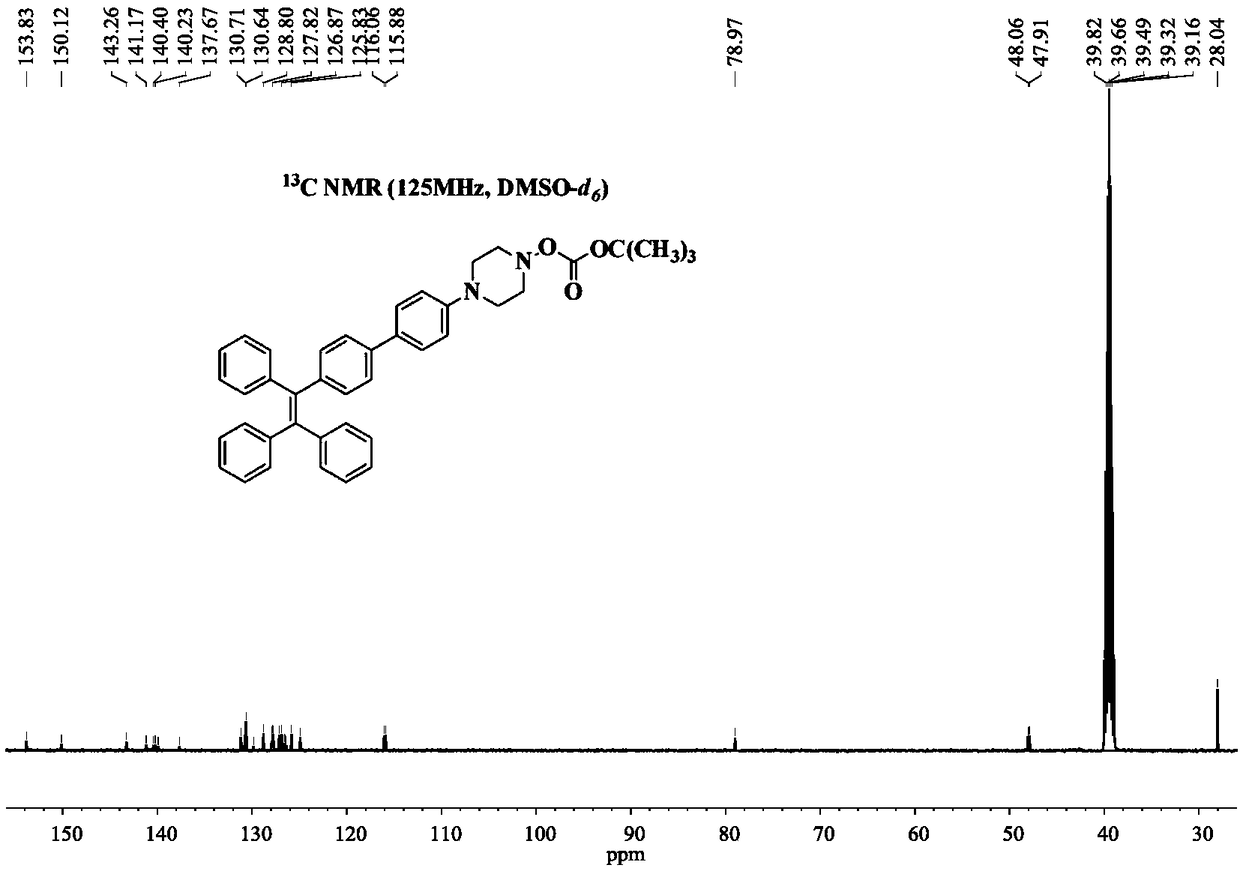
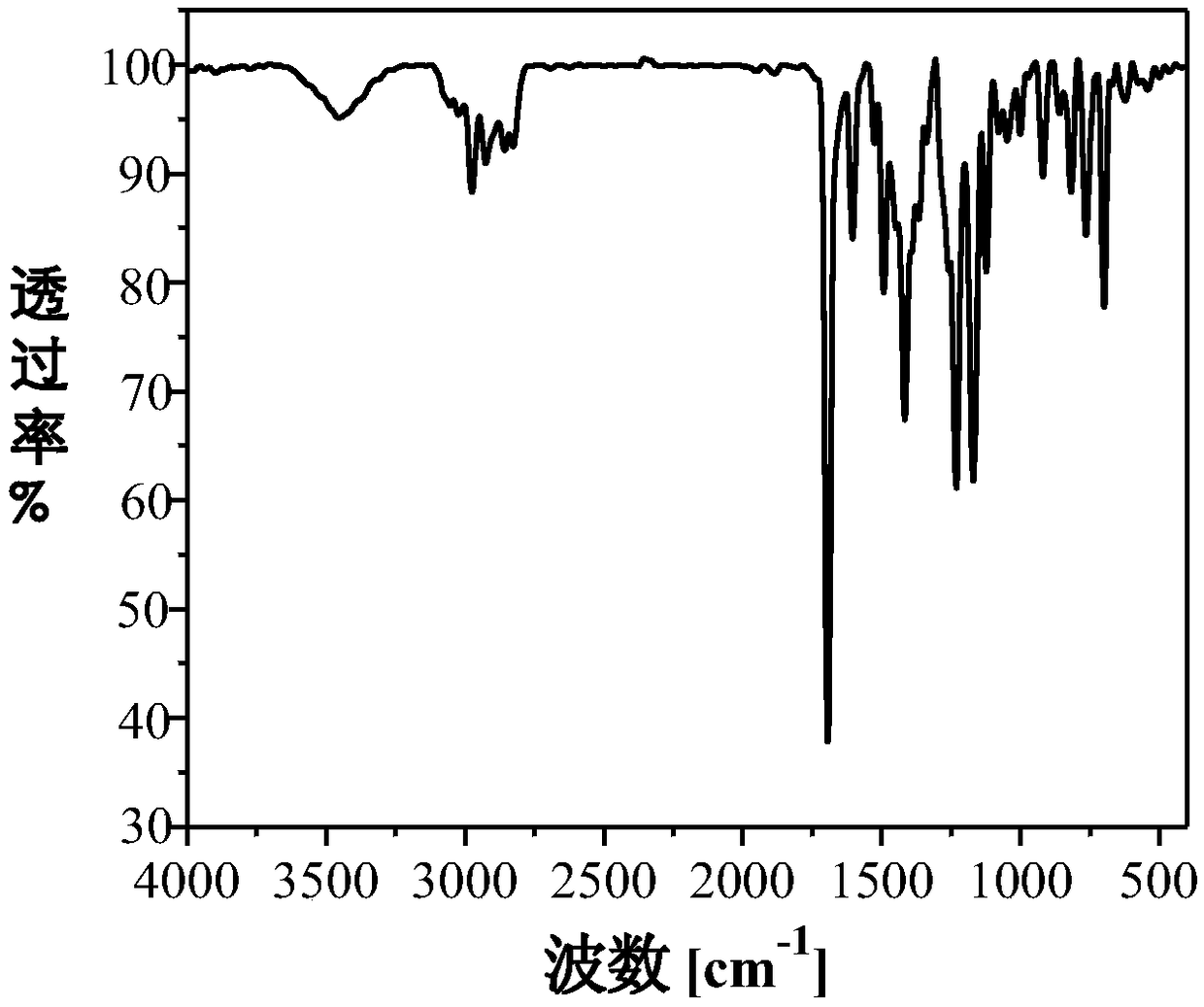
[0045] Example 1 Synthesis of 1-(4'-Boc-1-piperazinylphenyl)-1,2,2-triphenylethylene
[0046] Under the protection of high-purity nitrogen, 411mg of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, 485mg of 4-(4'-Boc-1-piperazinyl)phenylboronic acid Nacohol ester and 160mg of sodium carbonate (molar ratio 1:1.2:1.5) are mixed and dissolved in a mixed solvent prepared by 80mL of tetrahydrofuran and 20mL of water (volume ratio is 4:1), and after completely dissolving, add 6.2mg of Tetrakistriphenylphosphine palladium catalyst, the consumption of tetrakistriphenylphosphine palladium catalyst is 1.5wt% of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, reflux under 90 ℃ oil bath . After 12 hours of reaction, the oil bath was removed, and the system was naturally cooled to room temperature. Part of the organic solvent was removed with a rotary evaporator, and the mixture was concentrated to 1 / 3 of its original volume. Then extract the system with dichloromethane and saturated brine 60mL ...
Embodiment 2
[0047] Example 2 Synthesis of 1-(4'-Boc-1-piperazinylphenyl)-1,2,2-triphenylethylene
[0048] Under the protection of ordinary nitrogen, 411mg of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, 606mg of 4-(4'-Boc-1-piperazinyl)phenylboronic acid pinaca Alcohol ester and 202mg of potassium carbonate (molar ratio 1:1.5:1.5) are mixed and dissolved in a mixed solvent prepared by 100mL of acetonitrile and 20mL of water (volume ratio is 5:1). Triphenylphosphine palladium catalyst, the amount of four triphenylphosphine palladium catalyst is 2.0wt% of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, reflux under 100°C oil bath heating . After 10 hours of reaction, the oil bath was removed, and the system was naturally cooled to room temperature. Part of the organic solvent was removed with a rotary evaporator, and the mixture was concentrated to 1 / 3 of its original volume. Then the system was extracted with dichloromethane with a volume ratio of 1:2 and 60 mL of saturated saline for a tota...
Embodiment 3
[0049] Example 3 Synthesis of 1-(4'-Boc-1-piperazinylphenyl)-1,2,2-triphenylethylene
[0050] Under argon protection, 410 mg of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, 485 mg of 4-(4'-Boc-1-piperazinyl) phenylboronic acid pinaca Alcohol ester and 160mg of sodium carbonate (molar ratio 1:1.2:1.5) are mixed and dissolved in a mixed solvent prepared by 80mL of tetrahydrofuran and 20mL of water (volume ratio is 4:1), and after completely dissolving, add 6.2mg of tetrahydrofuran Triphenylphosphine palladium catalyst, tetrakistriphenylphosphine palladium catalyst used in an amount of 1.5wt% of 1-(4'-bromophenyl)-1,2,2-triphenylethylene, refluxed in an oil bath at 90°C. After reacting for 18 hours, the oil bath was removed, and the system was naturally cooled to room temperature. Part of the organic solvent was removed with a rotary evaporator, and the mixture was concentrated to 1 / 3 of its original volume. Then extract the system with 60mL of dichloromethane and saturated sali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com