Preparation method of lithium ion battery cathode material lithium vanadium phosphate
A lithium ion battery, lithium vanadium phosphate technology, applied in battery electrodes, phosphates, phosphorus oxyacids, etc., can solve the problems of long cycle and complex production process of lithium vanadium phosphate, and achieve energy saving and good cycle stability , the effect of high specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a preparation method of lithium vanadium phosphate lithium ion battery cathode material, the method comprising the following steps:
[0027] a. Liquid phase mixing reaction: dissolving lithium nitrate, ammonium metavanadate, ammonium dihydrogen phosphate, carbon source, amine organic matter and additives into water to obtain a solution;
[0028] The consumption of described lithium nitrate, ammonium metavanadate and ammonium dihydrogen phosphate is 3:2:3 by lithium, vanadium, phosphorus element meter molar ratio; The consumption of described lithium nitrate and carbon source is meter lithium and carbon element molar ratio 1:2-4; the molar ratio of the lithium nitrate to the amine organic matter is 1:1-3; the amount of the additive is 0.1-3% of the quality of the prepared lithium vanadium phosphate, and the additive is selected from ferric nitrate, nitric acid At least one of aluminum, chromium nitrate, and magnesium nitrate;
[0029] b. Preparati...
Embodiment 1
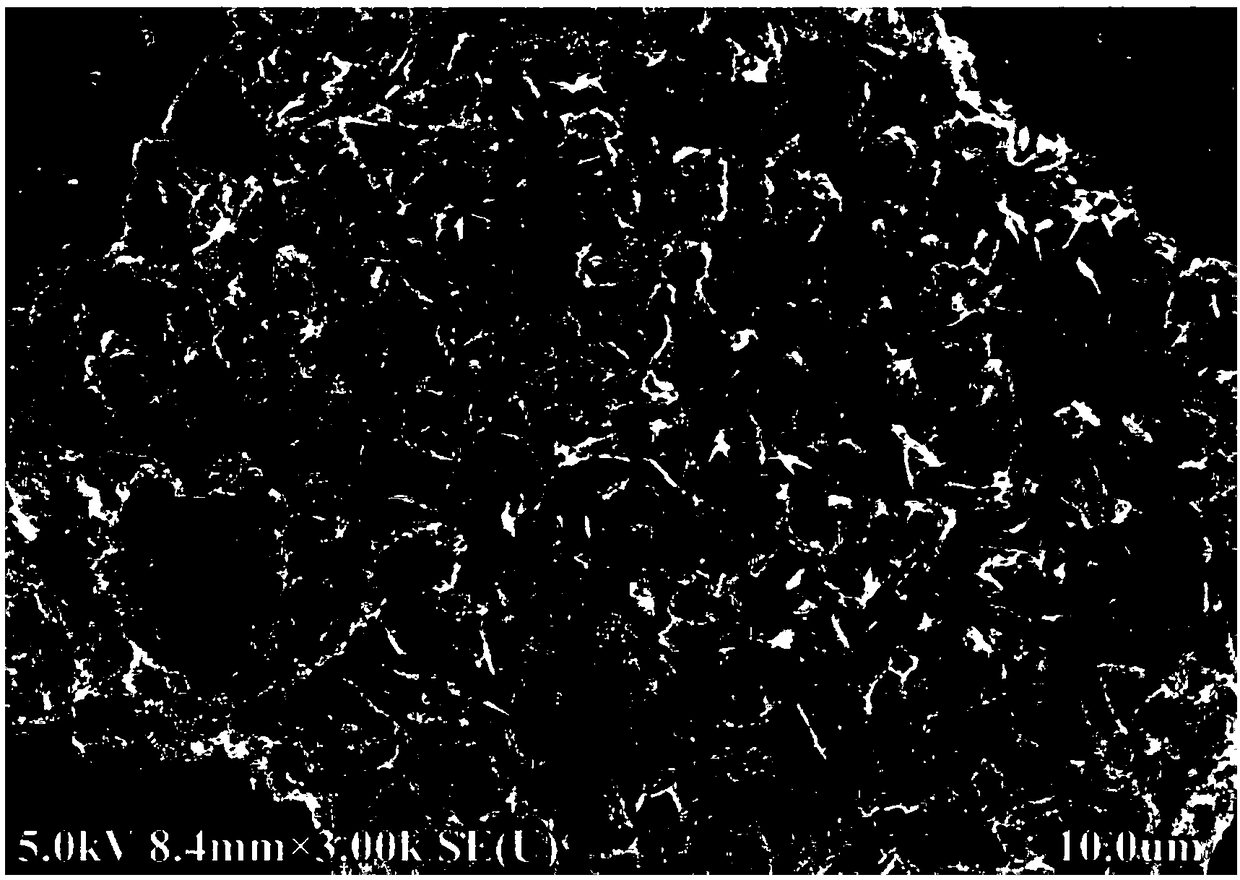
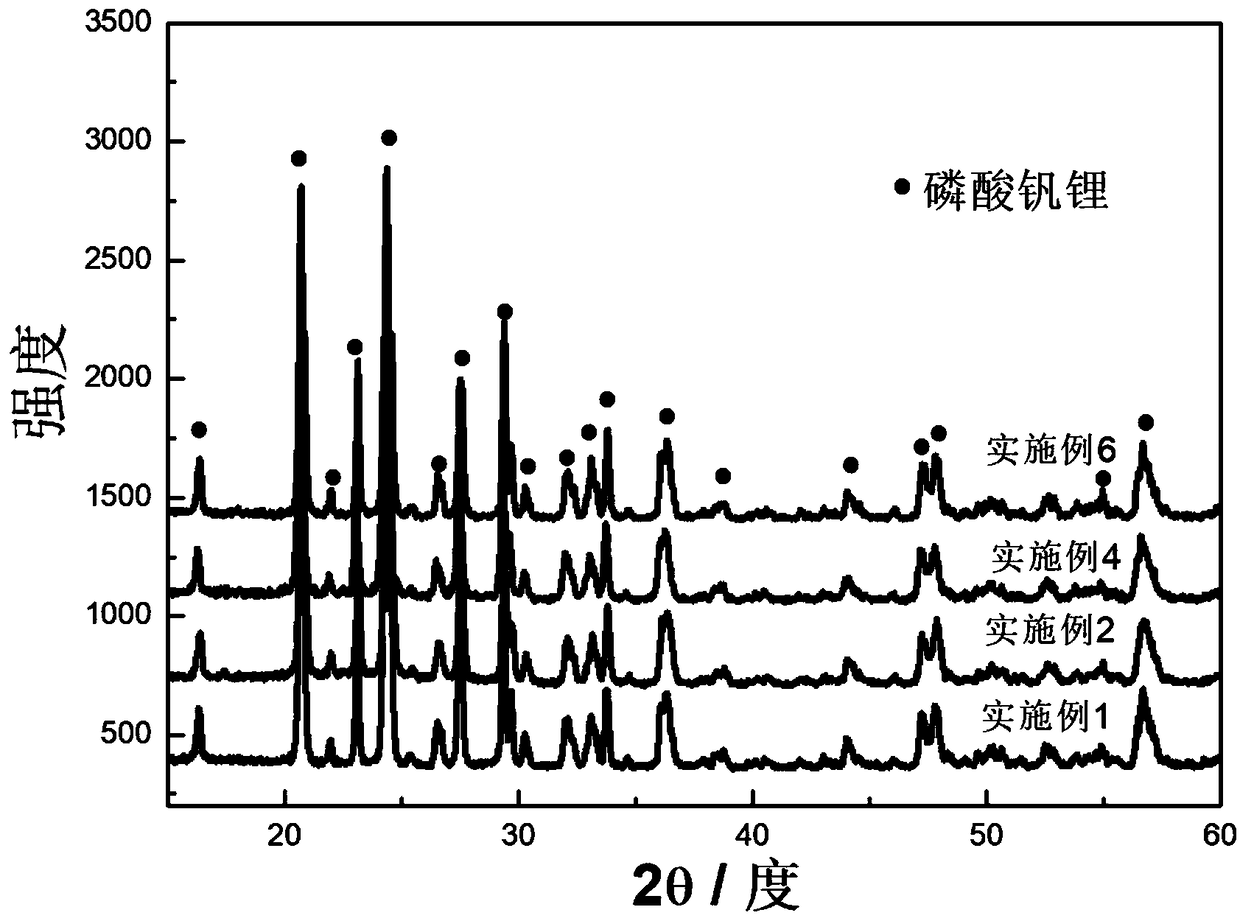
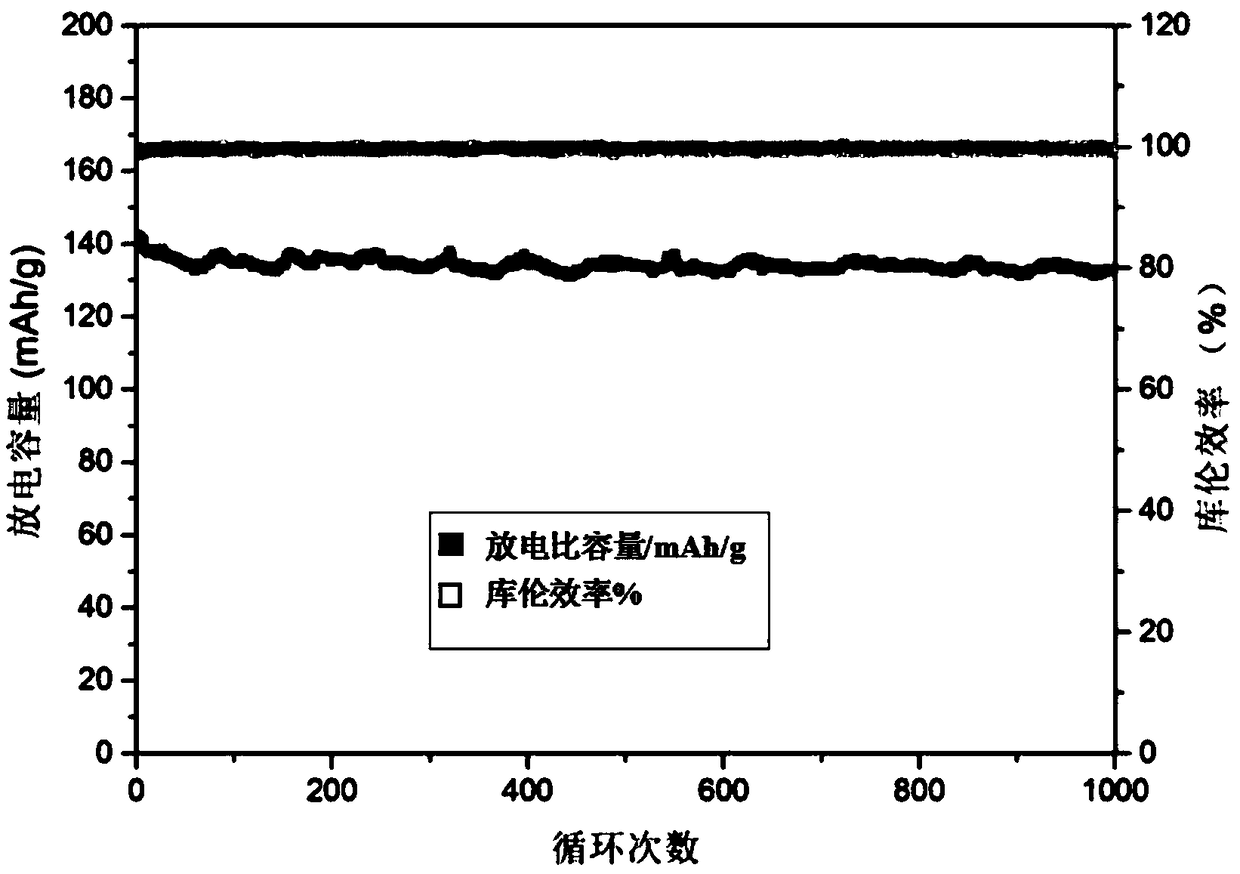
[0043] Dissolve 0.02mol ammonium metavanadate, 0.03mol ammonium dihydrogen phosphate, 0.03mol lithium nitrate, 0.06mol glycine, 2g glucose and 0.3g ferric nitrate in 150mL deionized water at room temperature to prepare a solution. Heating and stirring on a temperature-controlled electric furnace, heating to 100°C, the solution is volatilized and concentrated to form a colloidal substance, and the precursor powder is obtained after the reaction is completed, and the precursor powder is kept at 800°C under a nitrogen atmosphere for 4 hours to obtain a lithium-ion battery The positive electrode material is lithium vanadium phosphate. figure 1 It shows that lithium vanadium phosphate has a porous sheet structure, figure 2 It shows that the product has a good lithium vanadium phosphate structure, image 3 It shows that the first discharge capacity of the lithium battery performance test is 145mAh / g, and there is still a discharge capacity of 130mAh / g after 1000 cycles at a charge...
Embodiment 2
[0045] Dissolve 0.02mol ammonium metavanadate, 0.03mol ammonium dihydrogen phosphate, 0.03mol lithium nitrate, 0.07mol glycine, 2.5g citric acid and 0.4g ferric nitrate in 150mL deionized water at room temperature to prepare a solution. Heating and stirring on a temperature-controllable electric furnace, heating to 100°C, the solution is volatilized and concentrated to form a colloidal substance, and the precursor powder is obtained after the reaction is completed, and the precursor powder is kept at 700°C under an argon atmosphere for 6 hours to obtain Lithium vanadium phosphate lithium ion battery cathode material. Gained material structure is identical with embodiment 1, has porous sheet structure, figure 2 It shows that the product has a good lithium vanadium phosphate structure, Figure 4 It shows that the first discharge capacity of the lithium battery property test is 148mAh / g, and there is still a discharge capacity of 120mAh / g after 1000 cycles at a charge and disch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com