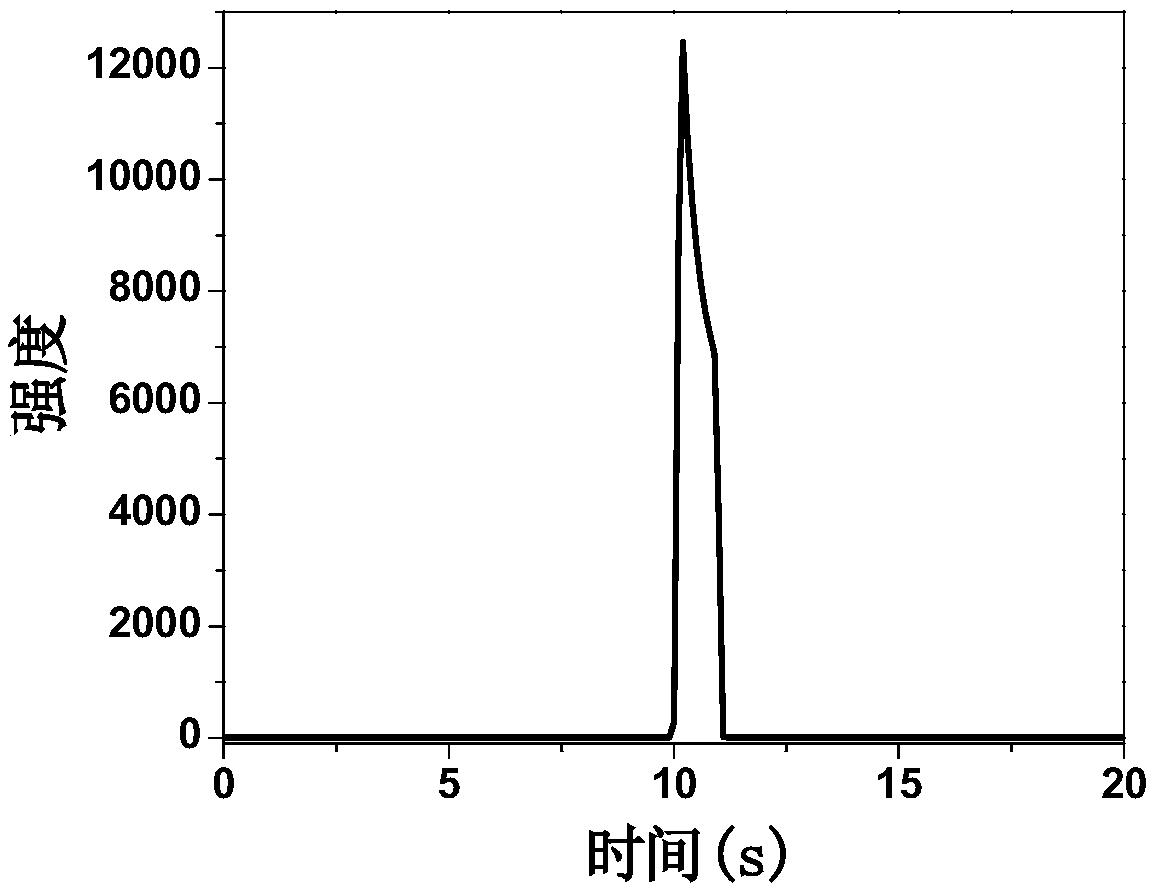
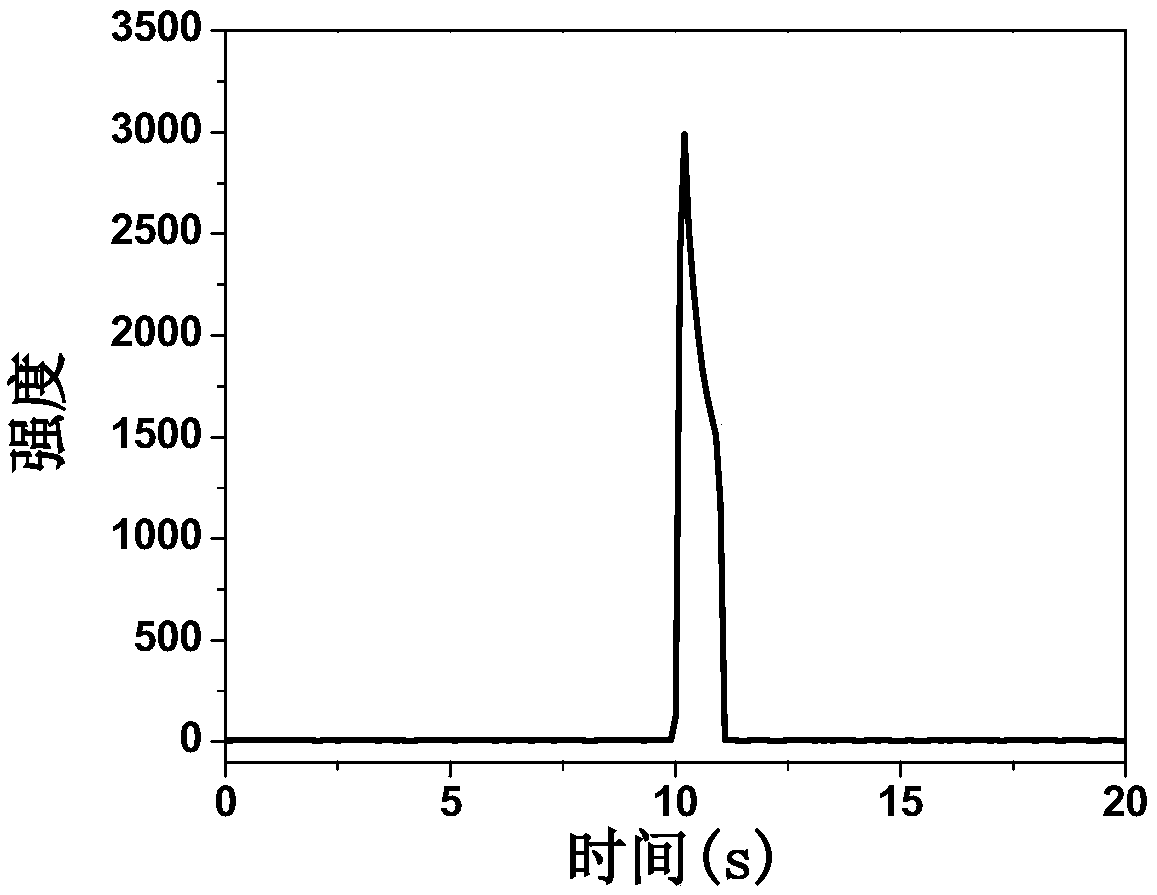
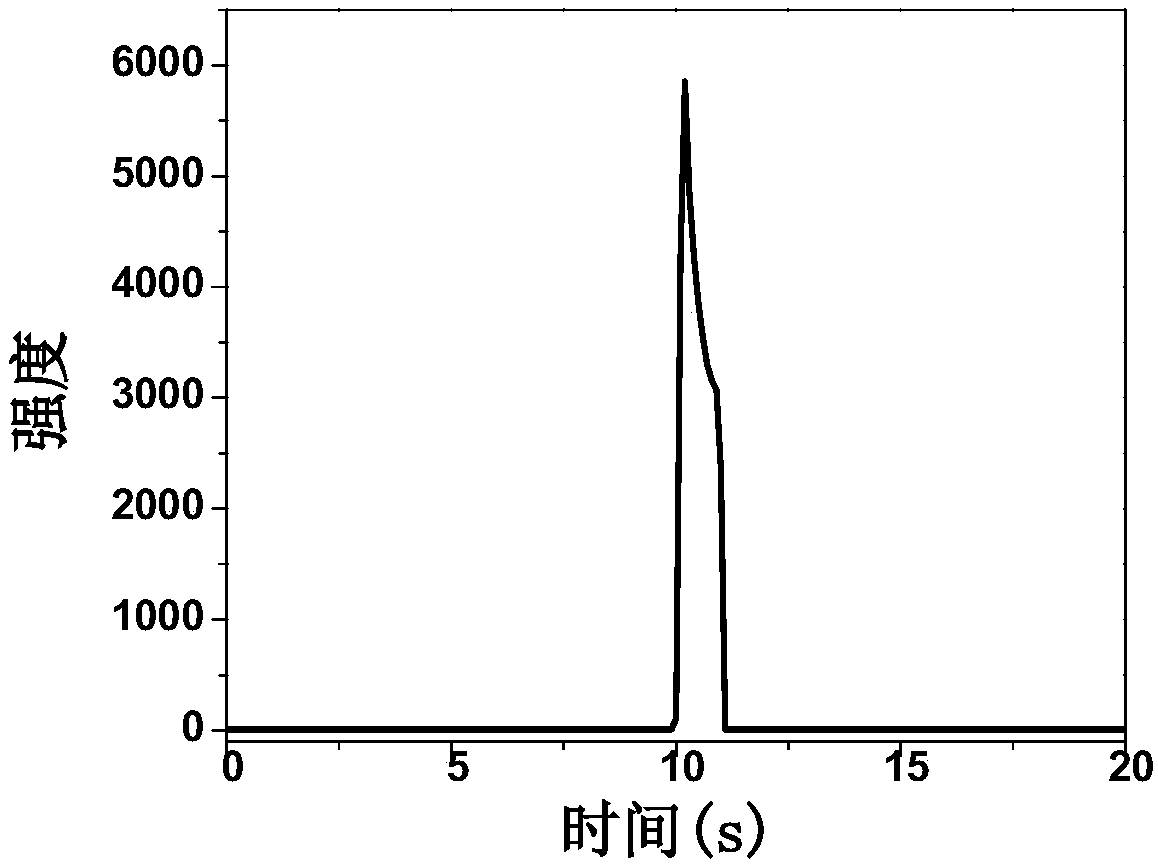
ECL (electrochemiluminescence) determining method for ATP (adenosine triphosphate)
A technology of adenosine triphosphate and adenosine triphosphate, which is applied in the field of adenosine triphosphate electrochemiluminescence determination, can solve the problems of expensive instruments, high sensitivity, and complicated operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 0.6 mL of 0.5 mol / L sodium hydroxide and 0.4 mL of 20 mg / mL chloroauric acid solution to 4 mL of 0.08 mol / L N-acetyl-L-cysteine solution, mix After uniformity, they were incubated in a constant temperature water tank at 37 °C for 3 h. After the reaction, the reaction solution was purified by dialysis with a dialysis bag with a molecular cut-off of 3500 to obtain an aqueous solution of N-acetyl-L-cysteine-gold nanoclusters, which was freeze-dried to obtain N-acetyl-L-cysteine Acid-gold nanocluster powder.
Embodiment 2
[0051] Glassy carbon electrodes with a diameter of 3 mm were coated with 1.0 μm, 0.3 μm and 0.05 μm Al 2 o 3 The powder is polished and polished in turn until it reaches a smooth mirror surface, and then put in HNO in turn 3 solution, absolute ethanol, ultrasonic cleaning in deionized water for 3 minutes, N 2 blow dry. Take 5 μL of the N-acetyl-L-cysteine-gold nanocluster solution prepared in Example 1 and drop it on the surface of the treated glassy carbon electrode, and dry it at room temperature to obtain N-acetyl-L-cysteine-gold nanocluster solution Gold nanoclusters modified glassy carbon electrodes. The N-acetyl-L-cysteine-gold nanocluster modified glassy carbon electrode was soaked in 0.1 mol / L sodium borohydride solution for 5 min to obtain the gold nanocluster probe modified electrode. The above-mentioned gold nanocluster probe modified electrode was used as the working electrode, the platinum wire was used as the counter electrode, and the Ag / AgCl electrode was u...
Embodiment 3
[0053] Glassy carbon electrodes with a diameter of 3 mm were coated with 1.0 μm, 0.3 μm and 0.05 μm Al 2 o 3 The powder is polished and polished in turn until it reaches a smooth mirror surface, and then put in HNO in turn 3 solution, absolute ethanol, ultrasonic cleaning in deionized water for 3 minutes, N 2blow dry. Take 5 μL of the N-acetyl-L-cysteine-protected gold nanocluster solution prepared in Example 1 and drop it on the surface of the treated glassy carbon electrode, dry it at room temperature, and further soak the electrode in 0.1 mol / L react in sodium borohydride solution for 5 minutes to obtain a gold nanocluster probe modified glassy carbon electrode. A three-electrode system was adopted, with gold nanocluster probe-modified glassy carbon electrode as the working electrode, platinum wire electrode as the counter electrode, and Ag / AgCl as the reference electrode. 4 -H 2 SO 4 (1:1 V / V) solution, manganese dioxide was electrodeposited, the deposition potential...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com