Composition, preparation method and application of oxygen-containing pidogrel optical isomer or salt thereof
A composition and compound technology, applied in the field of medicine, can solve the problems of preparation method research, lack of preparation technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
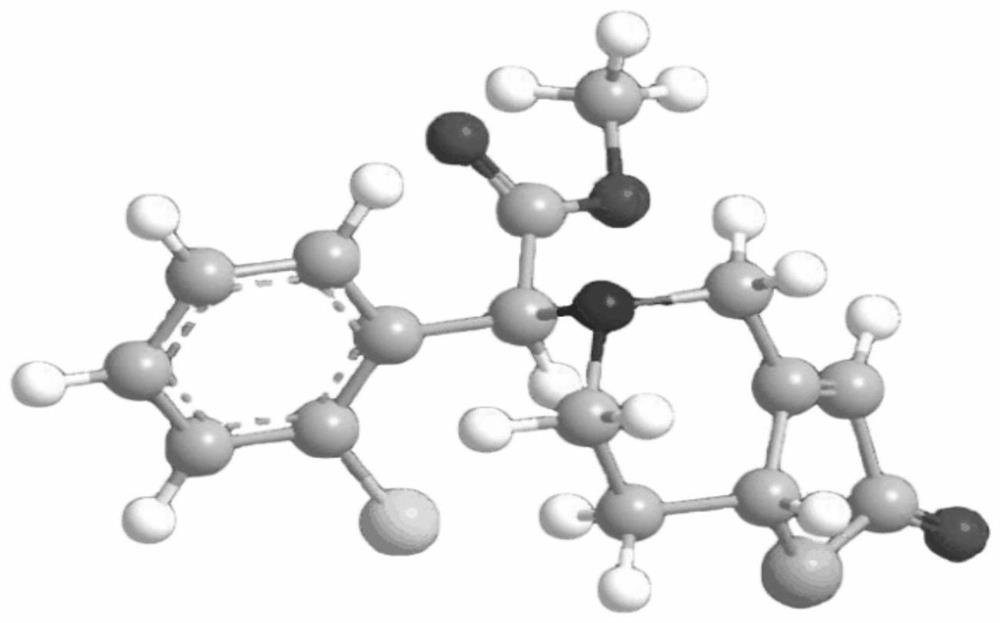
[0044] The preparation of embodiment 1 (7aR, 2S)-2-oxopidogrel and bisulfate
[0045] The first step: the synthesis of methyl o-chloromandelate p-benzenesulfonate
[0046]
[0047] Add 28g of p-nitrobenzenesulfonyl chloride, 0.3g of dimethylaminopyridine, 30g of methyl 2-chloromandelate and 300ml of dichloromethane into a 500ml three-necked flask at room temperature, add 20g of triethylamine, protect with nitrogen, and stir at room temperature for about 2 hours , TLC monitoring of the reaction until all the raw materials were converted, 400ml of dilute hydrochloric acid was added, the organic layer was separated, and concentrated under reduced pressure to obtain 30g of solid.
[0048] The second step: the preparation of the synthesis of (7aR,2S)-2-oxopidogrel
[0049]
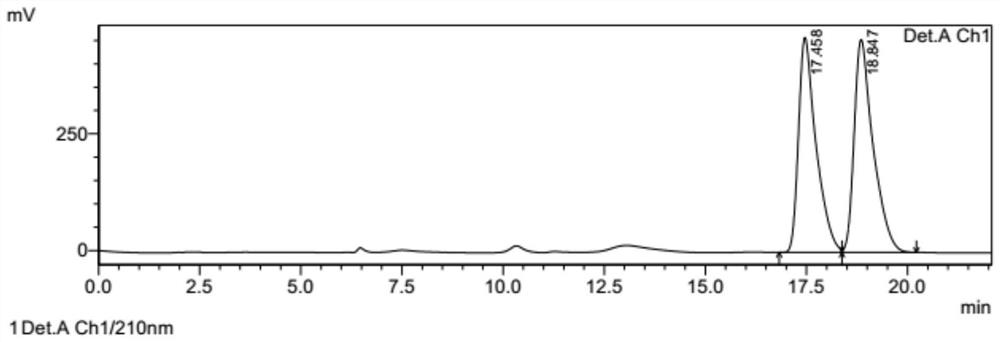
[0050] Add 0.5g of thienopyridine hydrochloride, 2g of sodium bicarbonate, 1g of sulfonate intermediate and 10ml of acetonitrile into a 100ml reaction flask, heat to 50°C for about 20 hours, monitor the ...
Embodiment 2
[0058] The preparation of embodiment 2 (7aR, 2S)-2-oxopidogrel hydrobromide
[0059]
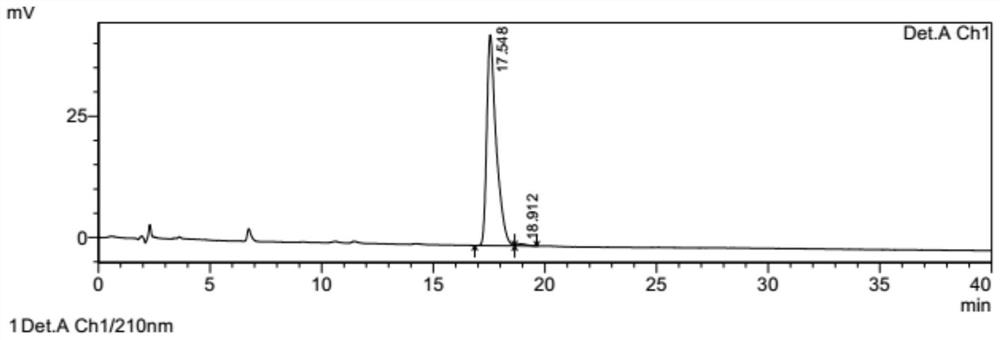
[0060] The preparation method of (7aR, 2S)-2-oxopidogrel is the same as in Example 1 above, dissolve 1 g of (7aR, 2S)-2-oxopidogrel in ethyl acetate, add hydrobromic acid dropwise to form a salt, and filter to obtain (7aR , 0.7g of crude product of 2S)-2-oxopidogrel hydrobromide.
[0061] Adding: add optically active (7aR, 2S)-2-oxopidogrel hydrobromide crude product into the reaction flask, heat to reflux with methanol, then add acetone, control the ratio of methanol to acetone to be 1:5;
[0062] Precipitation of crystals: Slowly lower the temperature to room temperature and stir for crystallization for 24 hours to obtain a white solid of (7aR,2S)-2-oxopidogrel hydrobromide.
[0063] MS m / z(ES):338.06[M+1]
[0064] 1H NMR (300MHz, DMSO), δ1.65~1.75(m, 1H), 2.45-2.52(m, 1H), 2.88~2.94(m, 1H), 3.02~3.05(m, 1H), 3.75(s, 3H), 3.90~3.97(m, 1H), 4.22~4.25(m, 1H), 4.61-4.65(m, 1H), 5.26(s, ...
Embodiment 3
[0065] The preparation of embodiment 3 (7aR, 2S)-2-oxopidogrel hydrochloride
[0066]
[0067] The preparation method of (7aR, 2S)-2-oxopidogrel is the same as in Example 1 above, dissolve 1 g of (7aR, 2S)-2-oxopidogrel in 10 ml of ethyl acetate, add dropwise concentrated hydrochloric acid to form a salt, and filter to obtain (7aR , 2S)-2-oxopidogrel hydrochloride crude product 0.7g.
[0068] Adding: add optically active (7aR, 2S)-2-oxopidogrel hydrochloride crude product into the reaction flask, heat to reflux with methanol, then add acetone, control the ratio of methanol to acetone to be 1:10;
[0069] Precipitation of crystals: Slowly lower the temperature to room temperature and stir for crystallization for 36 hours to obtain a white solid.
[0070] MS m / z(ES):338.06[M+1]
[0071] 1 H NMR (300MHz, DMSO), δ1.72~1.79(m, 1H), 2.42-2.53(m, 1H), 2.79~2.85(m, 1H), 3.04~3.08(m, 1H), 3.69(s, 3H), 3.93~3.95(m, 1H), 4.25~4.28(m, 1H), 4.61-4.65(m, 1H), 5.29(s, 1H), 6.44(s, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com