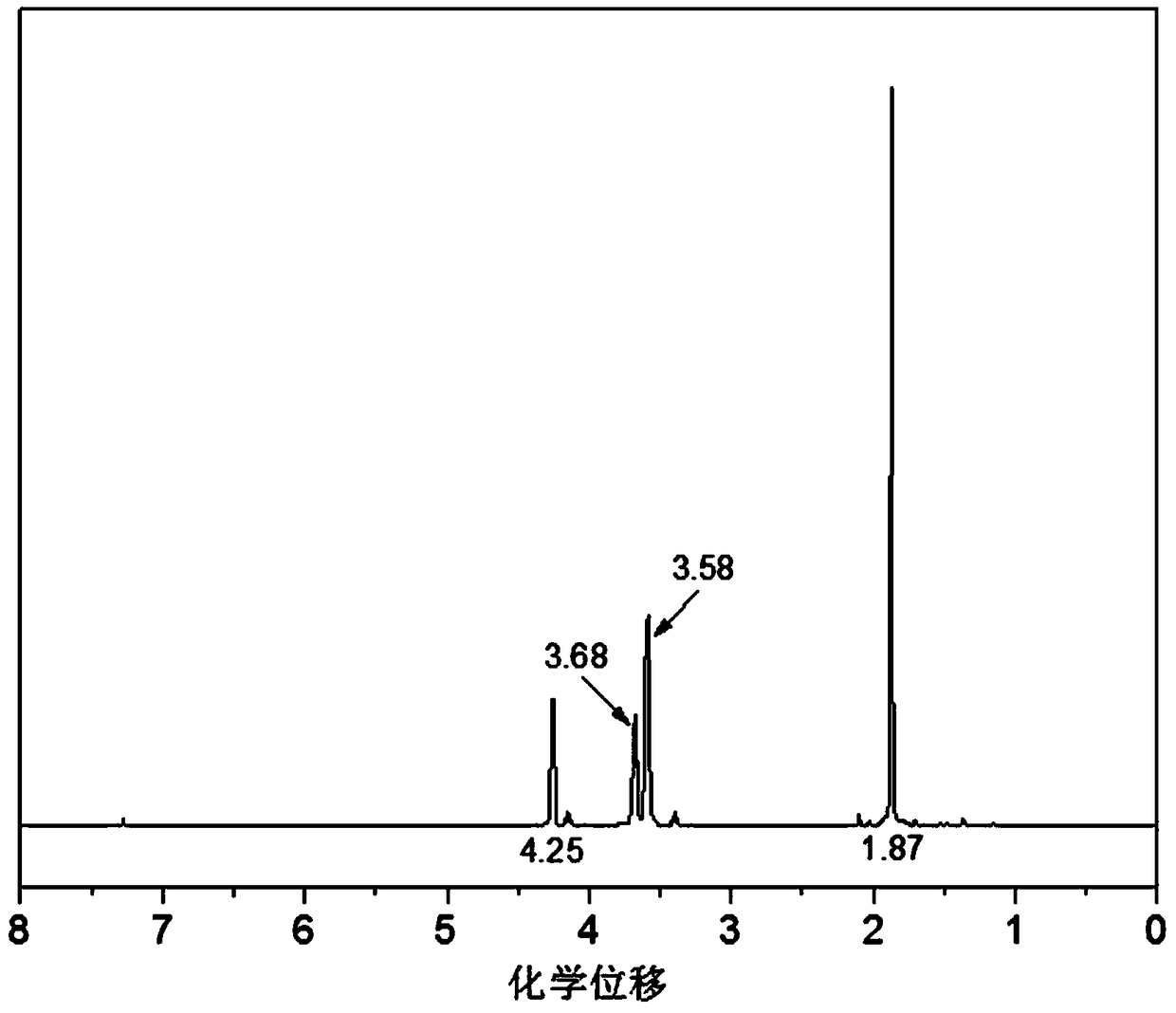
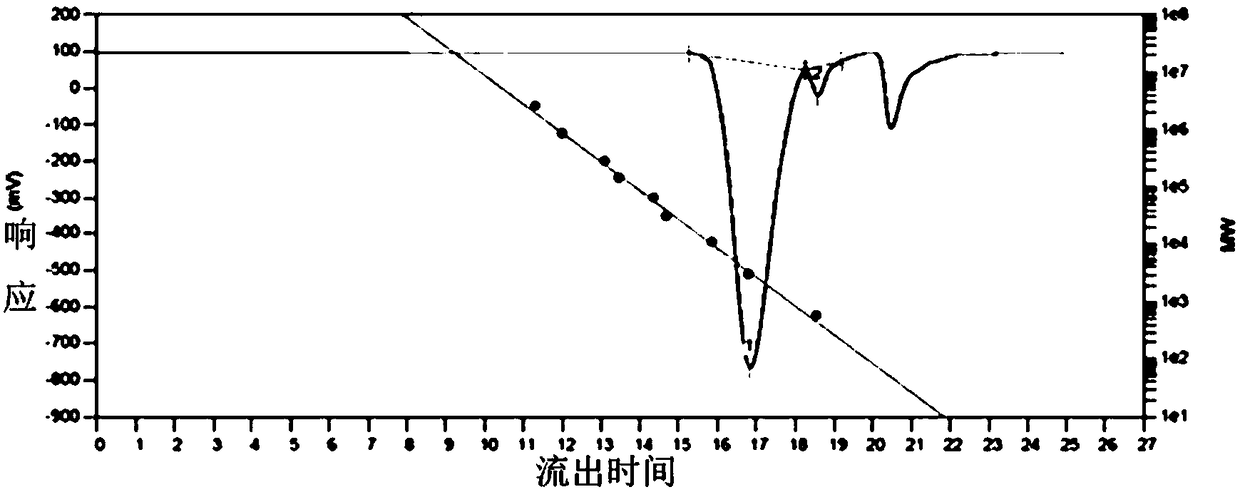
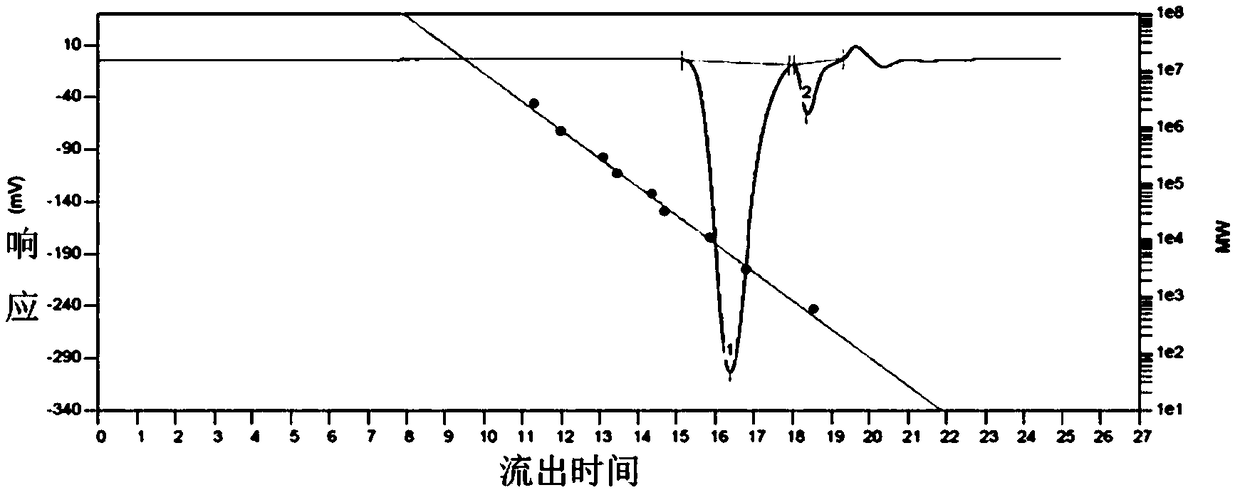
Preparation method of copolyether containing fluoroalkyl
A technology of fluorine-containing alkyl copolyether and alkyl copolyether, which is applied in the field of fine chemical synthesis technology and polymer materials, and can solve problems such as inability to prevent water and oil, low fluorine content of fluorine-containing groups, and insufficient surface energy. problems, to achieve good appearance, easy surface properties, and good comprehensive mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) One-pot preparation of fluorine-containing alkyl epoxy monomers
[0055] a. Synthesis of nonafluorooxetane
[0056] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.5g of enbutyl acetate, 26.6g of sodium bicarbonate (NaHCO 3 ), 55.0g sodium dithionite, 220g N,N-dimethylformamide, 210g water, 55.4g perfluorobutyl iodide was added dropwise, reacted at -15°C for 3h, slowly raised to room temperature, and kept for 2h. After the reaction was completed, extraction was performed three times with 150 g of toluene. The extracts were combined and washed three times with water to obtain a solution of 4-nonafluorobutyl-3-iodobutyl acetate, which was directly put into the next step of cyclization reaction without treatment.
[0057] The above-mentioned fluorine-containing alkyl iodide butyl acetate solution was directly added into a 1000 mL three-neck flask, 305 g of sodium hydroxide solution with a mass concent...
Embodiment 2
[0073] (1) One-pot preparation of fluorine-containing alkyl epoxy monomers
[0074] a. Synthesis of nonafluorooxetane
[0075] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 15.9g of butyl acetate, 27.1g of sodium bicarbonate, 55.3g of sodium dithionite, 218g of N,N-dimethylformamide, 209g of water, 54.9g of perfluorobutyl iodide was added dropwise, reacted at -12°C for 3h, slowly raised to room temperature, and kept for 3h. After the reaction was finished, extract with 165g trifluorotoluene in 3 times. The extracts were combined and washed three times with water to obtain a solution of 4-nonafluorobutyl-3-iodobutyl acetate, which was directly put into the next step of cyclization reaction without treatment.
[0076] The above-mentioned fluorine-containing alkyl iodide butyl acetate solution was directly added into a 1000mL three-neck flask, and 310g of sodium hydroxide solution with a mass concentration of 20%...
Embodiment 3
[0091] (1) One-pot preparation of fluorine-containing alkyl epoxy monomers
[0092] a. Tridecafluorooxetane Synthesis
[0093] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.8g of butyl acetate, 27.9g of sodium bicarbonate, 54.8g of sodium dithionite, 235g of N,N-dimethylformamide, 230g of water, 72.6g of perfluorohexyl iodide was added dropwise, reacted at -14°C for 5h, slowly raised to room temperature, and kept for 6h. After the reaction was finished, extract with 188g trifluorotoluene three times. The extracts were combined and washed three times with water to obtain a solution of 4-tridecafluorohexyl-3-iodobutyl acetate, which was directly put into the next step of cyclization reaction without any treatment.
[0094] The above-mentioned fluorine-containing alkyl iodide butyl acetate solution was directly added into a 1000mL three-necked flask, and 511g of sodium hydroxide solution with a mass concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com