Biocompatible boric acid nano-drug compound as well as preparation method and application thereof
A nano-drug, biocompatible technology, applied in the fields of material science and biomedicine, can solve the problems of ineffective nano-delivery carrier, slow metabolism, low drug-carrying rate, etc., achieve remarkable cancer treatment efficiency, high biological safety, High biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
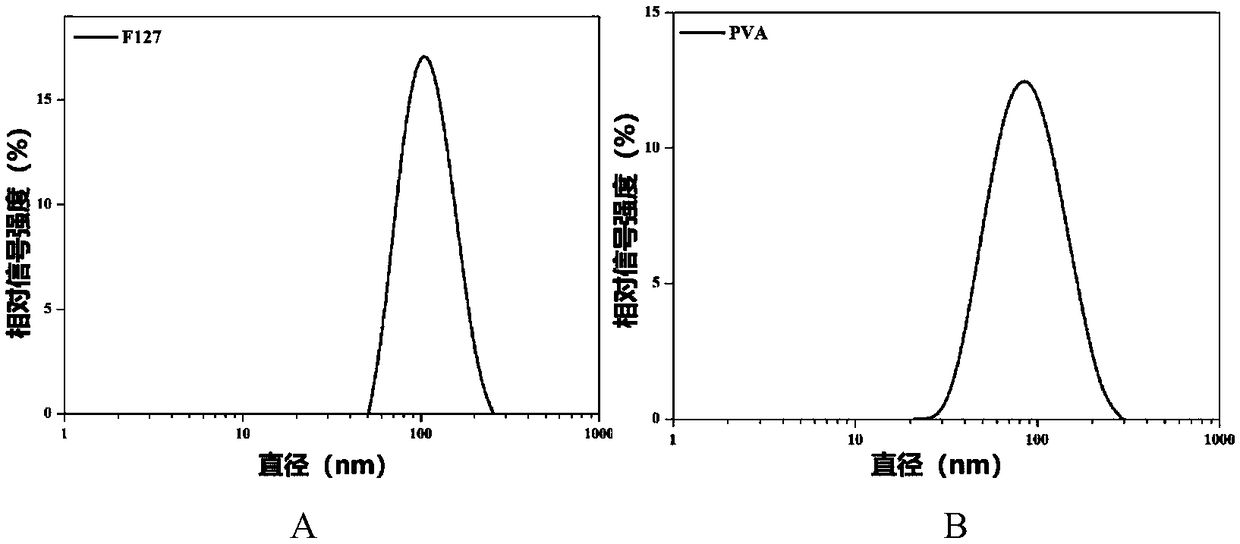
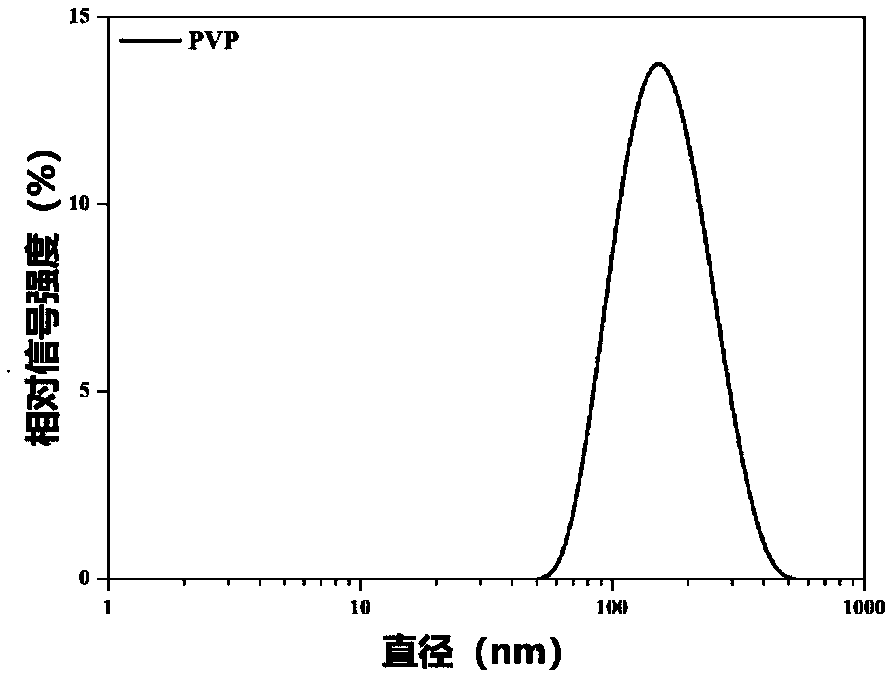
[0054] Example 1 This example involves the size comparison of BTZ / TA / F127, BTZ / TA / PVA and BTZ / TA / PVP and the morphology of BTZ / TA / PVP.
[0055] Bortezomib (BTZ) and tannic acid (TA) were mixed in dimethyl sulfoxide solution at room temperature (mass ratio 1:1.443), and then added dropwise to polyvinyl alcohol (PVA) and polyoxyethylene The aqueous solution of oxypropylene ether block copolymer (F127) was stirred for 10s to form BTZ / TA / F127 and BTZ / TA / PVA complexes. Then the BTZ / TA / F127 and BTZ / TA / PVA complexes were placed in phosphate buffer, and the particle size of the complexes was detected by dynamic light scattering.
[0056] Bortezomib (BTZ), tannic acid (TA) and polyvinylpyrrolidone (PVP) were mixed in dimethyl sulfoxide solution at room temperature (mass ratio 1:1.475:1.75), then added dropwise to water and stirred for 10 s to form BTZ / TA / PVP complex, the BTZ / TA / PVP complex was placed in aqueous solution and phosphate buffer solution respectively, and the size and shap...
Embodiment 2
[0060] Example 2 This example relates to the stability of the BTZ / TA / PVP complex in different physiological environment solutions over time.
[0061] The BTZ / TA / PVP complex prepared in Example 1 was respectively placed in water, phosphate buffer and 10% fetal bovine serum, and its stability over time under different physiological conditions was observed by dynamic light scattering.
[0062] Experimental results:
[0063] Such as Figure 4 Shown, the size change of BTZ / TA / PVP complex over time in water, phosphate buffer and 10% fetal calf serum, the results show that, over time, within 48 hours, BTZ / TA / PVP The complexes can exist stably in the different physiological environments mentioned above.
Embodiment 3
[0064] Example 3 This example relates to the release pattern of the bortezomib component in the BTZ / TA / PVP complex at different pH values.
[0065] The BTZ / TA / PVP complex prepared in Example 1 was dialyzed, and then the dialyzed BTZ / TA / PVP complex was packaged in a dialysis bag (molecular weight cut-off was 3500), and then placed in phosphoric acid with different pH In the buffer solution, the content of bortezomib in the phosphate buffer solution was regularly detected by high performance liquid chromatography (the elution ratio of bortezomib, methanol:water=7:3), and then its release curve was drawn.
[0066] Experimental results:
[0067] Such as Figure 5 Shown, under different pH, the release law of bortezomib monomer in BTZ / TA / PVP complex, the result shows, when pH is 7.4, bortezomib basically does not release; As pH approaches tumor microenvironment (pH = 6.5) and endosomes (pH = 5.0), the release of bortezomib components was greatly increased, indicating that the BTZ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com