A kind of preparation method of quasi-spherical lithium nickel manganese oxide positive electrode material
A technology of lithium nickel manganese oxide and positive electrode materials, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of uneven particle size of products and uneven local concentration of solutions, so as to simplify the production process and improve electrochemical performance , the effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
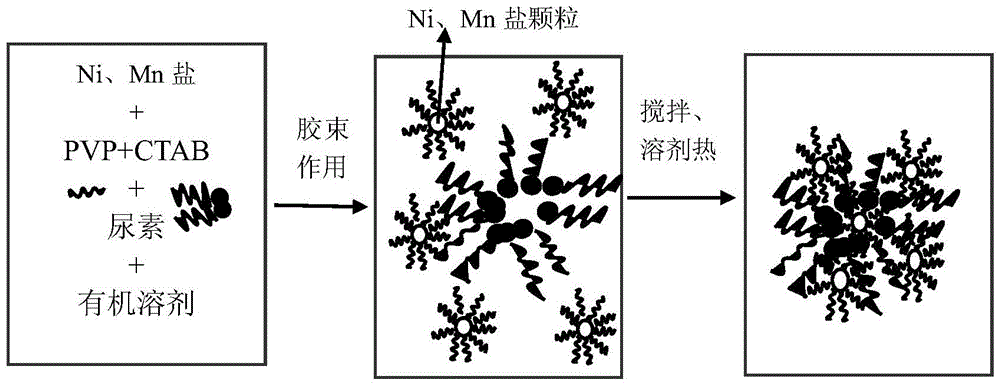
[0030] 6.22g (0.025mol) nickel acetate (Ni(CH 3 COO) 2 4H 2 O) and 12.98g (0.075mol) manganese acetate (Mn(CH 3 COO) 2 ) was dissolved in 50ml of ethanol, the total concentration of metal ions was 2.0M, and 15.02g (0.25mol) of urea was added according to the molar ratio of urea to metal ions of 2.5:1, and 0.08g of CTAB and 0.16g of PVP were added after magnetic stirring for 30min. The mixed solution was magnetically stirred for 2 hours, then transferred to a polyvinyl fluoride-lined autoclave, sealed and reacted at 160°C for 12 hours, cooled to room temperature naturally, centrifuged, washed, and dried to obtain Ni 0.25 mn 0.75 CO 3 Precursor. It looks like figure 2 As shown, it is flocculent-like spherical secondary particles composed of primary flake-like particles.
[0031] The resulting Ni 0.25 mn 0.75 CO 3 The precursor was pre-fired at 500 °C for 3 hours, and then combined with Li 2 CO 3 According to Li:(Ni+Mn)=1.03:2 (molar ratio), after mixing evenly, cal...
Embodiment 2
[0033] 6.54g (0.0225mol) nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 16.88g (0.0675mol) manganese nitrate (Mn(NO 3 ) 2 4H 2 O) Dissolve in 60ml of ethylene glycol, the total concentration of metal ions is 1.5M, add 8.11g (0.135mol) urea according to the molar ratio of urea to metal ions of 1.5:1, stir magnetically for 30 minutes, then add 0.05g CTAB and 0.15g PVP, the mixed solution was magnetically stirred for 2 hours, then transferred to a polyvinyl fluoride-lined autoclave, sealed and reacted at 165°C for 15 hours, naturally cooled to room temperature, centrifuged, washed, and dried , making Ni 0.25 mn 0.75 CO 3 Precursor.
[0034] The resulting Ni 0.25 mn 0.75 CO 3 The precursor was pre-fired at 500 °C for 3 hours, and then mixed with LiNO 3 According to Li:(Ni+Mn)=1.07:2 (molar ratio), after mixing evenly, calcining at 780°C in the air for 12 hours, after natural cooling and grinding, the LiNi 0.5 mn 1.5 o 4 Cathode material. Mix this material with acetylene ...
Embodiment 3
[0036] 4.98g (0.02mol) nickel acetate (Ni(CH 3 COO) 2 4H 2 O) and 10.38g (0.06mol) manganese acetate (Mn(CH 3 COO) 2 ) was dissolved in 80ml of ethylenediamine, the total concentration of metal ions was 1.0M, and 9.61g (0.16mol) of urea was added according to the molar ratio of urea to metal ions of 2:1, and 0.04g of CTAB and 0.12 g PVP, the mixed solution was magnetically stirred for 2 hours, then transferred to a polyvinyl fluoride-lined autoclave, sealed and reacted at 170°C for 10 hours, cooled to room temperature naturally, and the obtained precipitate was centrifuged, washed, and dried. Made Ni 0.25 mn 0.75 CO 3 Precursor.
[0037] The resulting Ni 0.25 mn 0.75 CO 3 After the precursor was pre-fired at 500 °C for 3 hours, it was mixed with LiOH·H 2 O is mixed uniformly according to Li:(Ni+Mn)=1.05:2 (molar ratio), calcined at 800°C in air for 12 hours, and the LiNi is obtained after natural cooling and grinding. 0.5 mn 1.5 o 4 Cathode material. Its X-ray d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com