Preparation method of alogliptin intermediate
A technology for intermediates and preparation steps, applied in the field of preparation of alogliptin intermediates, can solve problems such as limiting total yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of composite oxide
[0024] The preparation method is a hydrothermal method, specifically comprising the following steps:
[0025] Under vigorous stirring at room temperature, add 10mmol of zinc acetate, 5mmol of manganese acetate, 12g of polyvinylpyrrolidone and 8g of urea into a mixture of 120mL of deionized water and ethylene glycol and mix evenly, wherein: the volume ratio of deionized water to ethylene glycol The ratio was 2:1; after that, the obtained mixture was transferred to a stainless steel reaction kettle, and placed in a dry oven at 210°C for 20h. After the reaction kettle was cooled to normal temperature, it was filtered, washed, and samples were collected. Dry at 80°C for 12 hours in air, and calcined at 500°C for 4 hours to obtain a composite oxide.
[0026] The SEM characterization of the above composite oxide was observed on a JSM-7001F scanning electron microscope with a working voltage of 200kV. The powder sample was ...
Embodiment 2
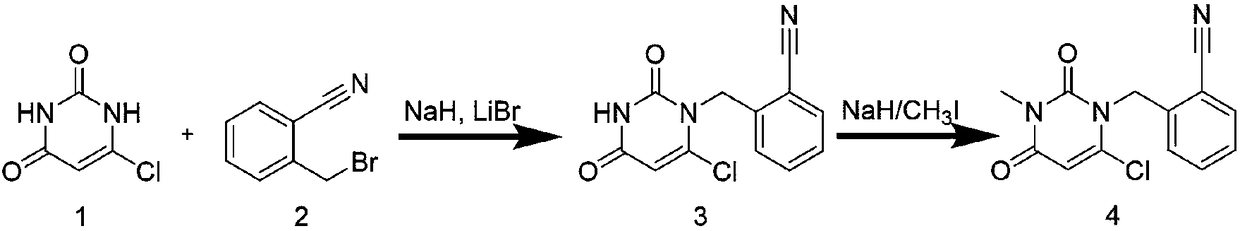
[0027] Embodiment 2: prior art prepares intermediate compound 3
[0028] The raw material compound 1 (10.0 g, 68 mmol) was added to 100 ml of DMSO / DMF mixed solvent with a volume ratio of 1:1, and stirred at room temperature to dissolve. After being dissolved, 2.5 g of sodium hydride and 2.5 g of lithium bromide were added. The raw compound 2 (13.4g, 68mmol) was dissolved in 30ml of DMSO / DMF mixed solvent with a volume ratio of 1:1, and the solution of compound 2 was slowly added to the mixed solution of compound 1 under stirring conditions, and the reaction was stirred for 12h. The reaction solution was filtered and concentrated to obtain a crude product. After purification by silica gel column chromatography (dichloromethane / acetone, 75:25 isocratic elution), the intermediate compound 3 was obtained.
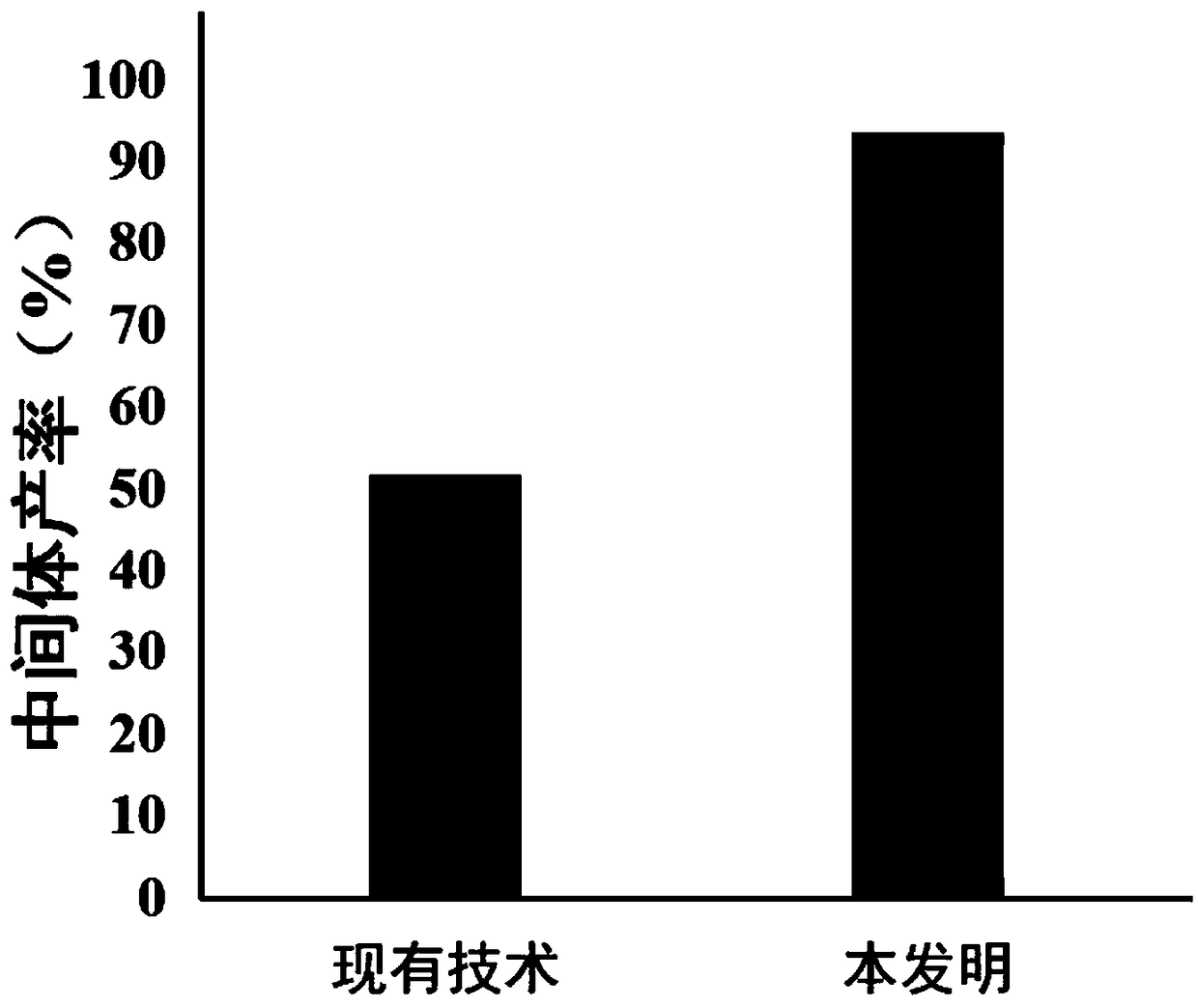
[0029] The dry weight of intermediate compound 3 was 9.2 g, and the yield was 51.7%.
Embodiment 3
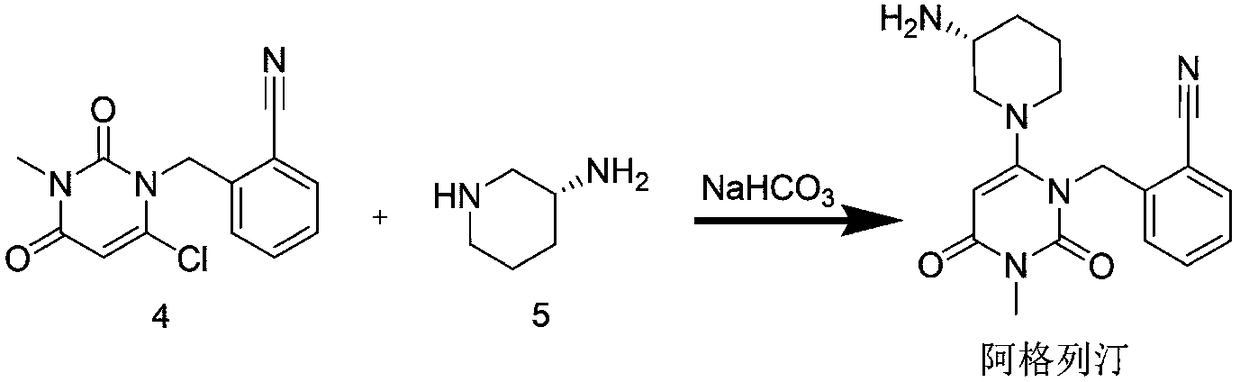
[0030] Embodiment 3: the inventive method prepares intermediate compound 3
[0031] The raw material compound 1 (10.0 g, 68 mmol) was added to 100 ml of DMSO / DMF mixed solvent with a volume ratio of 1:1, and stirred at room temperature to dissolve. After being dissolved, 2.5 g of sodium hydride, 2.5 g of lithium bromide and 5 g of the composite oxide prepared in Example 1 were added. The raw compound 2 (13.4g, 68mmol) was dissolved in 30ml of DMSO / DMF mixed solvent with a volume ratio of 1:1, and the solution of compound 2 was slowly added to the mixed solution of compound 1 under stirring conditions, and the reaction was stirred for 12h. The reaction solution was filtered and concentrated to obtain a crude product. After purification by silica gel column chromatography (dichloromethane / acetone, 75:25 isocratic elution), the intermediate compound 3 was obtained.
[0032] The dry weight of intermediate compound 3 was 16.5 g, and the yield was 93.5%.
[0033] The difference b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com